Abstract
Context
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic that erupted in December 2019 has affected more than a million people from over 200 countries, claiming over 70 000 lives (by April 7, 2020). As the viral infection is driven by increased angiotensin-converting enzyme-2 (ACE2) expression, with the kidney exhibiting the highest expression, it is crucial to gain insights into the mechanisms underlying renal cell carcinoma (RCC) and coronavirus disease 2019 (COVID-19).
Objective
This study considers up-to-date information on the biological determinants shared by COVID-19 and renal disease, and aims to provide evidence-based recommendations for the clinical management of RCC patients with COVID-19.
Evidence acquisition
A literature search was performed using all sources (MEDLINE, EMBASE, ScienceDirect, Cochrane Libraries, and Web of Science). As of March 31, 2020, the Center for Disease Control reported that of the adults hospitalized for COVID-19 with underlying conditions in the USA, 74.8% had chronic renal disease.
Evidence synthesis
Evidence is discussed from epidemiological studies on SARS-CoV-2 pandemic and molecular studies on the role of kidney in facilitating routes for SARS-CoV-2 entry, leading to increased virulence of SARS-CoV-2 and clinical manifestation of symptoms in RCC.
Conclusions
This analysis will advance our understanding of (1) the molecular signatures shared by RCC and COVID-19 and (2) the clinical implications of overlapping signaling pathways in the therapeutic management of RCC and COVID-19 patients.
Patient summary
Amid the coronavirus disease 2019 (COVID-19) pandemic, patients diagnosed with renal cell carcinoma and infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may receive complimentary treatment modalities to enhance therapeutic response.
Keywords: Viral infection, Renal tumors, Therapeutic targeting
Take Home Message
This review provides novel biological insights into coronavirus disease 2019 (COVID-19)-infected patients with renal disease, with special emphasis on renal cell carcinoma and development of science-based action guidelines and therapeutic strategies in the clinical management of renal cell carcinoma patients diagnosed with COVID-19.
1. Introduction
In December 2019, a novel coronavirus (nCoV) outbreak emerged in the city of Wuhan, China. By the beginning of February 2020, The World Health Organization (WHO) formally named the disease triggered by 2019-nCoV as coronavirus disease 2019 (COVID-19). The Internal Committee of Taxonomy of Viruses then named the disease severe respiratory syndrome coronavirus 2 (SARS-CoV-2), another β-coronavirus cluster related to severe acute respiratory syndrome (SARS) of 2003 and Middle East respiratory syndrome (MERS) of 2012 [1], [2]. As of April 7, 2020, the global pandemic had caused over 1.2 million cases and 72 000 deaths, with numbers rising each day [3].
While investigations are intensified as to the mechanisms of viral infectivity, person-to-person transmission of the virus includes droplet inhalation transmission and contact transmission through oral, nasal, and eye mucous membrane contacts [4]. Symptoms of virulence varies depending on the patient but often includes fever and dry cough, while others suffer from fatigue, dyspnea, nasal congestion, nausea, or diarrhea [5]. Cases can worsen and lead to acute respiratory distress syndrome (ARDS) or pneumonia. However, diagnosis is often complicated by a large portion of patients who are asymptomatic [6]. Those with pre-existing conditions, such as diabetes, hypertension, and pulmonary, cardiac, and kidney diseases are considered to be at a higher risk of developing severe disease [4], [7], [8], [9], [10]. Owing to the immense burden of the disease on the health, livelihood, and economics of the global population, it is crucial to consider the molecular aspects of the virus, its functional interaction with host cellular signaling, its association with certain comorbidities, and potential therapeutic strategies for the treatment of COVID-19.
As millions of cancer patients are fighting COVID-19, investigations into the incidence and clinical management of COVID-19 in patients with cancer, including renal cell carcinoma (RCC), are ongoing with intensity. This review aims to advance the current understanding of the impact of angiotensin-converting enzyme-2 (ACE2)-mediated SARS-CoV-2 infection on kidney function and the therapeutic targeting of underlying shared mechanisms of RCC and COVID-19.
2. Evidence acquisition
A literature search was performed using all sources (MEDLINE, EMBASE, ScienceDirect, Cochrane Libraries, and Web of Science). As of March 31, 2020, the Center for Disease Control (CDC) reported that, of the adults hospitalized for COVID-19 with underlying conditions in the USA, 74.8% had chronic renal disease.
3. Evidence synthesis
3.1. Mechanisms of SARS-CoV-2 infection
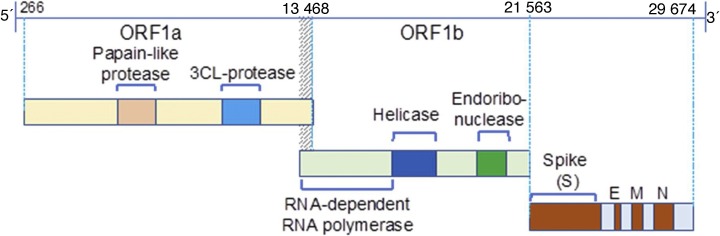
The novel zoonotic coronavirus (CoV) SARS-CoV-2 belongs to the subfamily Coronavirinae and genus Betacoronavirus. The 30 kb genome of SARS-CoV-2 is a single-stranded positive-strand RNA (+ssRNA; Fig. 1 ). Sixteen nonstructural proteins (NSPs; NSP1–16) derived from pp1a/pp1ab play specific roles in the replication of CoVs and in the formation of replication-transcription complex that facilitates synthesis of minus-strand messenger RNA called subgenomic RNAs (sgRNAs; Fig. 1). While NSPs are conserved among CoVs, there is diversity between structural proteins of CoVs, implicating their functional contribution to the infectivity, adaptation, and transmission. Based on phylogenetic and genomic analysis of the Betacoronavirus clusters, the SARS-CoV-2 is closely related to the β-coronaviruses bat‐SL‐CoV ZC45 and bat‐SL‐CoV ZXC2, and can cause infection of lower respiratory track and pneumonia in humans.
Fig. 1.
The SARS-CoV-2 genome. The 30 kb genome of 2019-nCOV is a single-stranded positive-strand RNA (+ssRNA). Only one-third of the genome serves as a template for four structural proteins that are functionally involved in the infection process. The four structural proteins include the membrane (M), spike (S), envelope (E), and nucleocapsid (N); all accessory proteins are derived from sgRNA and are critical for infection.
2019-nCOV = 2019 novel coronavirus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; sgRNA = subgenomic RNA.
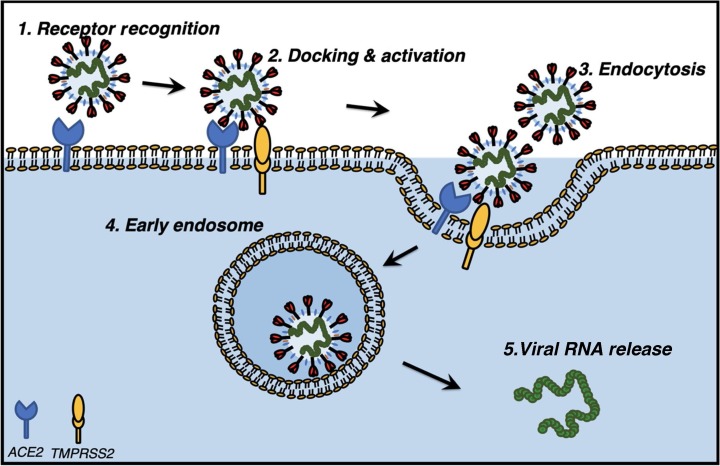
Structural Spike (S) proteins drive the entry of the CoVs SARS-CoV and SARS-CoV-2 into target host cells by engaging the cellular receptor ACE2. Analogous to SAR-CoV infection, interaction of the S protein of SARS-CoV-2 with cellular ACE2 facilitates the attachment of the virus to target cells [11]. This step is also functionally associated with the activation of cellular TMPRSS2, a type-II transmembrane serine protease that drives the entry of virus into the target cell and is regulated by androgens (Fig. 2 ). SARS-CoV infection is dependent on the proteolytic activity of TMPRSS2 and results in cleavage of SARS S protein at multiple sites [12]. Proteolytic cleavage of SARS S protein by TMPRSS2, known as S priming, mediates efficient virus-host cell fusion and decreases virus sensitivity to neutralizing antibodies [13].
Fig. 2.
Molecular pathway of SARS-CoV-2 activation in host cells. Mechanism of docking and internalization of SARS-CoV-2 into host cells are facilitated by host cellular proteins. Docking and host cell entry of SARs-CoV-2 occur via virion-associated “spike protein” recognition and binding with the ACE2 receptor (1). Receptor recognition and ACE2 activation are assisted by transmembrane protein TMPRSS2 (2), which leads to endocytosis of virions (3) and early endosome formation (4), and ultimately responsible for the release of viral RNA into the cytoplasm of host cells causing virulence.
ACE2 = angiotensin-converting enzyme-2; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TMPRSS2 = type-II transmembrane serine protease 2.
The ACE2 enzyme plays an important role in the renin-angiotensin system (RAS), as it counteracts the effects of angiotensin (Ang) II, a vasoactive peptide responsible for vasoconstriction and aldosterone release systemically. Ang I is converted to Ang II by ACE. However, ACE2 depletes Ang I and II levels by directly catalyzing the compounds and converting Ang I to Ang 1–9 and Ang II to Ang 1–7, known vasodilators acting through receptor Mas (MasR) with antifibrotic, antiproliferative, and anti-inflammatory effects [7], [14], [15], [16], [17], [18]. Angiotensin-converting enzyme inhibitors (ACEis), which block the conversion of Ang I to Ang II, and angiotensin receptor blockers (ARBs), which block the downstream interaction of Ang II with the Ang II type I (AT1) receptor, are used to treat hypertension and other cardiovascular diseases [19]. While these treatments do not directly modulate ACE2, they cause an increase in ACE2 expression [20]. Other medications, such as thiazolidinediones and ibuprofen, are speculated to increase ACE2 levels also [21]. Further, ACE2 levels are elevated in patients with multiple cardiovascular conditions, diabetes, and hypertension, those who are at a greater risk of mortality in COVID-19 [15], [21], [22]. Thus, RAS inhibition may affect COVID-19 outcomes by decreasing the proinflammatory activity of Ang II or increasing the virulence of the virus in the heart and lungs due to the increased ACE2 expression [10].
Both ACE2 and TMPRSS2 are coexpressed on ciliated bronchial epithelial cells and type II pneumocytes, epithelia of small intestine, and podocytes and the border of proximal tubule cells of the kidney, thus making these organ sites easy routes for SARS-CoV and SARS-CoV-2 infection [23]. In particular, ACE2 expression is higher in the kidneys than in any other organ, with potentially 100 times higher expression than in the lungs [24]. Podocytes and proximal straight tubule cells of the kidney share similar homology and have highly enriched signatures for kidney disease–related signaling pathways [23]. It is therefore of critical importance to investigate the effects of SARS-CoV-2 on patients with prior kidney issues as well as assess the potential risk of developing kidney injury once infected with COVID-19.
3.2. Epidemiology of COVID-19 and cancer
The COVID-19 pandemic created unique circumstances and challenges for cancer patients, including patients with renal cancer and their clinical providers across the clinical care continuum and management trajectory (ie, cancer diagnosis, treatment, follow-up care, end of life). Epidemiological evidence so far indicates that cancer patients have a higher risk of contracting COVID-19, developing complications, and deteriorating more rapidly [25]. Specifically, the mortality rate is significantly higher among SARS-CoV-2–infected patients with active cancer (28.6%) than in patients with other comorbidities. Older cancer survivors are more vulnerable to COVID-19 because of existing chronic, comorbid health conditions (diabetes, cardiovascular diseases, and respiratory diseases) [26]. Cancer patients presenting at clinics and hospitals for cancer care might have increased exposure to other infected patients and clinical care personnel, leading to increased risk of COVID-19, morbidity, and mortality.
To reduce cancer patients’ risk of exposure, difficult clinical decisions have to be made by physicians about for whom, how, and when to provide cancer treatment and follow-up care [27]. However, delays in cancer management might lead to missed opportunities for curative treatment and increased risks of cancer progression to metastasis, anxiety, and stress. For patients experiencing metastatic or recurrent cancer, considerations should include how such delays may lead to immediate need for hospital-based palliative care and symptom management [27]. For patients receiving treatment with chemotherapy or immunotherapy, or those who have undergone recent surgery, the risks are even higher because of the reduced immune system function. Reinforcement of the strict “stay at home” policy, although efficient in reducing the risk of exposure and contamination, is likely to increase patient clinical care (eg, managing disease and comorbidities), financial (eg, loss of job and medical insurance), and psychosocial (eg, social isolation, fear of contamination, stress, and anxiety) needs, thus worsening the quality of life and emotional well-being in a population already burdened by cancer and treatment impact.
To address some of these needs, several hospitals and clinics in the USA have adopted a telehealth care delivery approach; however, such shifts in care delivery approaches may pose significant challenges, especially for patients with limited access to Internet or computer skills [28]. For patients participating in cancer clinical trials, forced stay home or quarantine complicates hospital attendance for repeat appointments and continuity in care, and when severe complications or emergencies occur, treatment delays or unavailability will have significant implication for the patient’s health [29]. For renal cancer patients and patients with chronic renal disease in need of blood transfusion or kidney or transplant, care has to be taken to ensure the safety of blood or donor’s organs [30].
3.3. Surgical management of RCC amid COVID-19
The goal of surgical treatment for RCC is twofold: the first is the removal of the renal tumor with negative surgical margins via either partial or radical nephrectomy based on the tumor characteristics of size and location. This can be accomplished via an open or minimally invasive (robotic) approach. The second goal is the preservation of renal function. Most guidelines agree that partial nephrectomy should be performed for the renal mass when feasible to maximally preserve kidney function and prevent end stage renal disease. The COVID-19 pandemic has transformed the way we are approaching the surgical treatment of kidney cancer. The recommendations to be followed to reduce this risk to hospital workers are summarized in Table 1 .
Table 1.
Clinical recommendations to reduce transmission during renal surgery.
| Surgical patient triage | Depending on local transmission patterns and hospital needs, lower-risk kidney tumors should be postponed, while larger and aggressive tumors should be treated as the risk of progression must be weighed against the risks of COVID-19. |
| COVID-19 testing | All patients planning to undergo surgery for kidney cancer should be tested prior to surgery, depending on local community access to testing. |
| COVID-19–positive patients | If the patient is COVID-19 positive, every effort should be made to delay surgery until full recovery of the patient and viral shedding risk is reduced. |
| Operating room personnel | Limit personnel in the operating room during surgery, allowing only essential personnel, and limit traffic in and out of rooms. |
| PPE | PPE is mandatory and should include N-95 masks to mitigate transmission risk. |
| Operating room risk reduction | Efforts should be made to reduce transmission during intubation and extubation, with only ESSENTIAL personnel present during these times. In addition, surgical transmission via surgical plume should be reduced by lowering cautery settings, application time, and total duration of tissue desiccation. |
| Special considerations for minimally invasive surgery | During minimally invasive surgery, CO2 pressure should be maintained as low as safely possible, and gas leak or release from ports during surgery should be minimized. Every effort should be made to suction any residual CO2 at the end of procedure prior to tumor extraction. |
| A closed insufflation system should be used to reduce escape of CO2 into the OR. Filters vary in size; the smallest filter available should be implemented to the suction system. |
COVID-19 = coronavirus disease 2019; OR = operating room; PPE = personal protective equipment.
3.4. COVID-19 impacts the kidney
3.4.1. Chronic renal injury
As of March 31, 2020, the CDC reported that of the adults hospitalized for COVID-19 with underlying conditions in the USA, 74.8% had chronic renal disease, but patients with chronic renal disease consisted of only 3% of total cases [26], [31]. A study from Washington found that of 24 patients admitted to the intensive care unit (ICU), five (21%) had chronic kidney disease [32]. In the Lombardy region of Italy, of 1591 ICU patients with COVID-19 from 72 hospitals, 36 (3%) had chronic kidney disease. Various studies from Wuhan, China, investigating the early stages of the pandemic found that 2–4% of COVID-19 patients had chronic renal failure [8], [25], [33]. However, in a lager study of 1099 cases from mainland China, only 0.7% of patients had chronic renal failure [5].
Others have cautioned against the risk of COVID-19 spread in hemodialysis (HD) centers [34], [35], [36]. In the USA specifically, 0.5 million individuals receive dialysis treatment and constitute a high-risk group for adult respiratory distress syndrome [36]. Basile et al [37] studied a single HD center in Renmin Hospital, Wuhan University. The authors found that 37 of 230 patients on HD and four of 33 staff members developed infections in a 1-mo span from mid-January to -February. Patients on HD with COVID-19 had less lymphopenia, lower serum levels of inflammatory cytokines, and milder clinical disease than other patients with COVID-19 infection. Although six patients on HD died from the CoV infection, the presumed causes of death were not related to pneumonia but rather to cardiovascular diseases, cerebrovascular issues, and hyperkalemia [37]. The emerging recommendations are that dialysis patients infected with COVID-19 should continue with their regimen and stay at their current center, in order to minimize the risk to other patients and healthcare personnel [34].
3.4.2. Acute kidney injury
In the previous SARS and MERS-CoV epidemics, acute kidney injury (AKI) developed in 5–15% of cases and had a high mortality rate (60–90%) [34], [38], [39]. Kidney injury was also associated with an increased risk of death in patients with influenza A virus subtype H1N1 [40]. Similar results have been found for the current SAS-CoV-2 pandemic. In a postpartum study of patients from Wuhan from January to the beginning of March, Diao et al [9] showed that 27.06% (23/85) patients exhibited acute renal failure. However, another study conducted in a similar time frame in a hospital in Wuhan did not have anyone to develop or die of acute renal failure [33]. Several studies in China have tracked the development of AKI once admitted for COVID-19. A smaller study of 41 patients found that thee (7%) developed AKI. Other studies in Wuhan have found rates around 3–4% in a similar time frame [6], [25]. One study of 1099 cases from mainland China showed that six (0.5%) COVID-19 patients developed AKI [5].
A recent clinical study with 701 patients from a hospital in Wuhan found that 5.1% of patients admitted for COVID-19 developed AKI [8]. The same study also reported that on admission, 43.9% of patients had proteinuria, 26.7% had hematuria, and 13–14% had elevated serum creatinine, elevated blood urea nitrogen (BUN), and estimated glomerular filtration rate under 60 ml/min. The study confirmed that elevated baseline serum creatinine, elevated baseline BUN, AKI stage, proteinuria, and hematuria were independent risk factors for in-hospital death. The incidence of AKI was significantly higher in patients with elevated baseline serum creatinine; 33.7% of these patients died in hospital, a significantly higher percentage than that of patients with normal creatinine levels. Computed tomography scans of kidneys also showed reduced density, suggestive of inflammation and edema [8].
Further evidence confirmed overlap of COVID-19 patients with renal dysfunction. In two hospitals in Wuhan, 59% of patients with COVID-19 had proteinuria, 44% had hematuria, 14% had increased BUN levels, and 10% had increased serum creatinine [24]—data that were validated in another hospital [33]. Individuals who developed AKI had a 5.3 times higher mortality risk than those without AKI, higher than that of comorbid chronic illness (1.5 times more) [24]. Clinical evidence from Shanghai revealed that ICU patients were more likely to have increased creatinine (15.8% vs 4.1%) and BUN (26.3% vs 5.7%) [41].
The high ACE2 expression in the kidney and the presence of fragments of CoV in the patient blood and urine implicate COVID-19 in the renal system [33], [42]. Clinically, proteinuria in a large number of patients can be caused by virus-inducted cytopathic effect on podocytes, as podocyte injury induces heavy proteinuria [43]. Increased creatinine levels could also indicate indirect effects on renal function caused by the virus, such as hypoxia, shock, and rhabdomyolysis [4], [8]. Specifically, the effects of CoV-infected ACE2 can cause kidney damage through a heightened inflammatory response by depleting Ang 1–7 and Ang 1–9 levels. A systemic cytokine storm–induced inflammatory response can also cause multiple organ damage [42], [44]. It is unlikely that kidney injury arises from deposition of immune complex of viral antigen or virus-induced specific immunological effector mechanisms because glomeruli microscopy imaging has appeared normal in COVID-19 patients [38].
3.4.3. Renal cancer
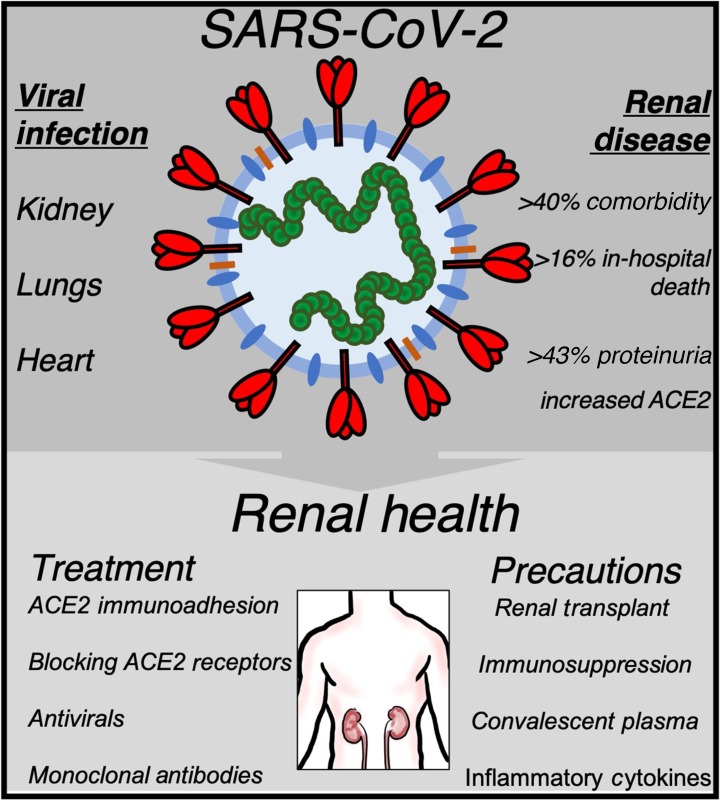
In addition to chronic and acute renal injury, renal cancer must also be assessed when evaluating the risk factors of developing severe COVID-19 infections. In the USA, nearly 60 000 individuals had RCC in 2018, although few studies have evaluated the incidence of renal cancer in COVID-19 cases [45]. Liang et al [46] found that 18 of 1590 cases had a history of cancer in hospitals throughout China, a rate higher than that of cancer in the overall Chinese population. Lung cancer was the most frequent type of cancer in the study population (28%), but one case had a history of RCC. Additionally, patients with cancer had a higher risk of severe complications than patients without cancer; cancer patients treated with chemotherapy or surgery had a higher risk of severe events than those not receiving treatment. These are important considerations in decision making regarding the clinical care of a cancer patient infected with the virus (Fig. 3 ).
Fig. 3.
SARS-CoV-2 infection in renal disease and therapeutic targeting of RCC. The top panel shows an overview of the primary signaling targets of viral infection and their association with renal disease. The bottom panel shows a proposed schema of the current therapeutic management (blocking ACE2 receptor pathway and hence viral internalization into host cells) and prevention strategies (controlling inflammation and immunosuppression, and consequently cell response to virulence) for COVID-19 patients with underlying renal disease.
ACE2 = angiotensin-converting enzyme-2; COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
3.5. Therapeutic management of RCC and COVID-19
3.5.1. Tyrosine kinase inhibitors and immune checkpoint inhibitors
A majority of cases with RCC are associated with the gene inactivation of von Hippel-Lindau, which normally regulates the levels of hypoxia-inducible factors. With the inactivation of this gene, the result is a subsequent downstream upregulation of hypoxia-inducible factors, which then causes angiogenesis and proliferation, mostly through the activation of the vascular endothelial growth factor receptor (VEGFR) [47]. Antiangiogenesis treatment of RCC includes the use of monoclonal antibodies that bind and deplete VEGF ligand, or bind to the VEGFR and tyrosine kinase inhibitors (TKIs) that block the intracellular domain of VEGFR. TKIs, specifically sunitinib and paxopanib, were first to be approved for frontline treatment of metastatic RCC (mRCC) [48].
The use of immune checkpoint inhibitors (ICIs) has become increasingly popular in the treatment of RCC. ICIs work to prevent the downregulation of cellular immune responses within tumors by targeting the interaction of programmed cell death protein (PD-1) and its ligand (PD-L1) and CTLA-4 and its ligand B7-CTLA-4 [49]. The standard of care of mRCC following the approval of the Food and Drug Administration (FDA) and the European Medicines Agency is the combination of a TKI and an ICI, such as nivolumab plus ipilimumab, which provides the best response rate and survival [50], [51], [52]. There have also been proposals for other combinations, such as pembrolizumab plus axitinib and avelumab plus axitinib [53]. The efficacy of ICIs in conjugation with COVID-19 infection is under intense investigation. Recent evidence suggests that restoration of immunocompetence with ICIs impacts the development of cytokine release syndrome and contributes to the severity of COVID-19 and ARDS [54]. Additionally, CoV-related interstitial pneumonia could be worsened by potential pneumological toxicity from anit-PD-1/PD-L1 ICI agents. However, discontinuation of ICIs in patients with COVID-19 infections is not recommended due relatively better outcomes than in those patients who are immunosuppressed from chemotherapy [46], [54]. In one case study, tocilizumab was used successfully to treat immune-related adverse events (irAEs), namely arthritis, in patients receiving ICI therapy. Tocilizumab, a monoclonal antibody against the interleukin (IL)-6 receptor, serves as a potential neutralizing antibody in treating COVID-19 [34]. This is of particular significance in the treatment of RCC patients with COVID-19 infection, as the treatment of ICIs and tocilizumab may overcome irAE side effects.
3.5.2. ACE inhibitors
RAS blockades, such as ACEis and ARBs, can potentially reduce cancer growth, lessen metastatic potential, and increase patient survival in RCC [55], [56]. Lower expression of Ang II receptors yielded better survival in patients with clear cell RCC, and increased ACE activity has been associated with higher tumor grades [57], [58]. While the decrease in degradation of bradykinin by ACEis may stimulate growth, survival, and migration of cancer cells, these effects must ultimately be offset by the decrease in angiogenic effects through the decreased VEGF expression due to decreased Ang II [55], [56]. There is also the potential for Ang 1–7, increased with the use of ACEis, to have antitumor effects by decreasing cell migration and invasion of cancer cells and reducing tumor growth by decreasing VEGF [59], [60], [61]. Mechanistically, the therapeutic use of ACEis in RCC has significant implications in the treatment of RCC patients who are infected with COVID-19 (Fig. 2). There is the potential to increase an RCC patient’s susceptibility to a more severe form of the disease due to increased ACE2 to which the virus can bind with the administration of ACEis.
3.5.3. Endothelin receptor antagonists
Endothelin receptor antagonists (ERAs) have been developed for the treatment of various cancers including renal cancer. The drugs decrease the effects of endothelin peptide isoform 1 (ET-1), which increases human tumor growth via activation of epidermal growth factor receptor, and direct angiogenic effects on endothelial cells through autocrine and paracrine pathways. In this way, ERAs have been shown to be effective cancer therapeutics [62]. ET-1 affects the kidney by binding to ETA and ETB G protein-coupled receptors, inducing sodium retention, inflammation, and fibrosis [63]. By mitigating these effects, ETA receptor antagonists such as ERAs have been shown to be promising agents in the treatment of chronic kidney disease as well [62]. Consequential to its vasoconstrictive action, the endothelin axis usually affects portal arterial hypertension (PAH), and ERAs are a common treatment against PAH by reducing the vasoconstrictive effects of ET-1 [62]. There is then the potential of ERAs to help those with hypertension to defend themselves against COVID-19.
3.6. Stratification and screening COVID-19 patients using liquid biopsy
“Liquid biopsy” is a minimally invasive procedure and a powerful tool in obtaining a profile of the tumor pathology [64], [65], [66]. Exosomes, cell-free (cf) DNA/RNA, and circulating tumor cells (CTCs) are currently at the forefront of liquid biopsy research, while cf proteins and lipids are also gaining momentum, and various biosensing assays are being developed for their analyses [67], [68]. Recent studies in RCC patients have utilized blood-based assays in combination with microRNA and/or protein biomarkers to provide tumor monitoring at high sensitivity and specificity [69]. Hence, RCC-associated biomarkers, which are found in circulating bodily fluids, are strong indicators of an unequivocal future of NextGen-liquid biopsy as novel tools for disease monitoring, biomarker discovery, and precision medicine. Recently, RCC-based liquid biopsy was validated and approved by the US FDA for the prognosis and diagnosis of the disease [70]. RCC-associated tumor-based CTCs and cfDNA are studied for their genomic and epigenomic landscape, providing vital information about tumor heterogeneity and tissue lineage [70]. Furthermore, two recent studies of several urinary- and serum-associated protein and RNA biomarkers in RCC, carbonic anhydrase IX (CAIX) and ceruloplasmin (CP), were found to be significantly increased in RCC patient’s urinary exosomes while they remain the same in the control cohort [71]. Podocalyxin-like protein 1 (PODXL; expressed in podocytes) and aquaporin 1 (AQP1, a water channel) are proteins expressed by kidney and not significantly increased in RCC [71]. More recent evidence identified serum miR-378 and miR-451 as RCC biomarkers [69].
Evidence on SARS-CoV-2 assessment suggests that urine contains almost no detectable COVID-19 viral RNA load, and only a small amount of viral RNA copies are present in blood (current tool of diagnosis is a nasopharyngeal swab followed by a reverse transcriptase polymerase chain reaction) [72]. However, elevated serum creatinine, BUN, and hematuria shown during hospitalization of COVID-19 patients are associated with higher mortality, pointing to an indirect mechanism of kidney damage in COVID-19–infected patients [8]. This evidence supports that liquid biopsy–assisted stratification and screening of COVID-19 patients with pre-existing RCC must focus on monitoring RCC biomarkers before and after COVID-19 infection.
3.7. Treatment options to cure COVID-19
For several of the patients with renal disease and AKI, treatment course proceeds similarly to that of most COVID-19 cases, although we are following a learning curve. General management including quarantining, use of protective personal equipment, nutritional and fluid support, ICU admission when necessary, and other treatments for complications are essential for all cases. Continuous renal replacement therapy (CRRT) has been shown to be effective in those with renal issues as well as other patients for SARS, MERS, and sepsis [38], [73]. CRRT is also being used in current cases of COVID-19 [33], as are glucocorticoids, which when administered in low doses improved outcomes for SARS and MERS; however, these drugs at high doses are associated with high mortality due to inhibition of viral clearance and prolongation of the duration of viremia [34], [74]. Additional therapeutic options effective in the treatment of COVID-19 patients are discussed below [75].
3.7.1. Monoclonal antibodies
An important therapy in combating SARS-CoV-2 is a neutralizing antibody directed against the S protein of the virus (Fig. 1). There exists a neutralizing monoclonal antibody directed against the Ras-binding domain of the S protein of MERS-CoV, suggesting the potential to discover a similar antibody for SARS-CoV-2 [76]. There have been promising preliminary results for tocilizumab, a monoclonal antibody against the IL-6 receptor, but more work is necessary to understand the safety and efficacy of the antibody [34]. The development of neutralizing antibodies poses an issue in rapid treatment strategy, as traditional screen options may be too slow for the pandemic to ensure sufficient breadth of treatment [75].
3.7.2. Oligonucleotides
Targeting the SARS-CoV-2 viral RNA genome for degradation may hold a promise, but it is challenging during the current pandemic, considering that viral RNA sequence domains are not known and limited targeted delivery of the oligonucleotide to the lungs may limit the scale-up manufacturing of such drugs for a large population of infected individuals [75].
3.7.3. Repurposing current antivirals
The Chinese National Health Commission recommends interferon-α and lopinavir/ritonavir, HIV protease inhibitors, in the treatment of COVID-19, as these treatments have had some clinical efficacy in treating both SARS and COVID-19 [34], [77]. Remdesivir, a drug that interacts with the viral polymerase, is clinically effective in treating MERS in mouse models and was effective in the treatment of a COVID-19 patient in the USA [75], [78]. Further research efforts focus on drugs that inhibit endocytosis, as clathrin-dependent endocytosis mediates the entry of SARS-CoV-2 into cells. A low pH and pH-dependent endosomal cysteine proteases cathepsins also facilitate the entry of the virus [79]. Thus, lysosomotropic agents, such as the antimalaria drugs chloroquine and hydroxychloroquine, can accumulate intracellularly and neutralize the endosome-lysosomal acidic pH, blocking protease activity and inhibiting viral entry into the cell (Fig. 2). Endosome-lysosomal protease inhibitors such as E64d or chlorpromazine, a clathrin-mediated endocytosis inhibitor, can also block endocytosis directly [80].
3.7.4. Convalescent plasma antibody transfer
As tried during the 2014–2015 Ebola outbreak, the use of the plasma of a recovered patient can provide polyclonal antibodies to different viral antigens of SARS-CoV-2, which can help treat patients who contract the virus later by providing a neutralizing effect and increasing the immune response of the patient. However, there are limitations in the speed and scope of such a development [75].
3.7.5. Blocking ACE2 receptors
A potential new strategy for drug development involves blocking the ACE2 receptor to inhibit the binding and subsequent entry of SARS-CoV-2 into cells. An antibody binding to the ACE2 protein blocks the entry of SARS effectively. Additionally, a small receptor-binding domain from the SARS S protein, which binds to the ACE2 receptor, has been shown to be effective in blocking the entry of SARS in cell culture [81].
3.7.6. ACE2 immunoadhesion strategy
A novel approach involves binding to the CoV itself through a soluble version of the ACE2 receptor, fused to an immunoglobulin Fc domain (ACE2-Fc) that binds to the S protein of SARS-CoV-2 and neutralizes the virus (Fig. 1). This methodology would allow for maximal breath, maximally engage the immune system to build lasting immunity, and decrease ACE2 levels in the lungs during infection, thus impacting acute respiratory distress pathophysiology [75]. Testing for the efficacy and safety of this novel therapeutic strategy is necessary, prior to clinical implementation.
3.8. Future directions
Through repurposing existing drugs such as ACEis and developing new antibodies to combat SARS-CoV-2, future clinical trials in COVID-19 patients battling RCC are a top priority in overcoming the pandemic (Fig. 3). Mechanistic exploitation of the baseline expression of ACE2 and TMPRSS2 in the lung epithelium and kidney tissue of RCC patients will lead to their therapeutic targeting and correlation with the therapeutic response. Whole-blood, whole-urine genomics have yet to be investigated in COVID-19 patients; of immediate clinical significance will be a study of cfDNA, CTCs, exosome-based biomarker’s correlation, and their genomic landscape among RCC and COVID-19–positive patients to enable new insights into the downstream effect of viral damage on renal function. Liquid biopsy could be the most effective tool for early identification, intervention, and improved prognosis among RCC patients harboring SARS-CoV-2 infection. As research efforts escalate globally toward the eradication of COVID-19 in cancer patients, our team joins the battle, driven by science-based evidence to develop action guidelines and therapeutic strategies in the management of RCC patients diagnosed with COVID-19.
4. Conclusions
This analysis will advance our understanding of the molecular signatures shared by RCC and COVID-19, and the clinical implications of overlapping signaling pathways in the therapeutic management of RCC and COVID-19 patients.
Author contributions: Natasha Kyprianou had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kyprianou, Badani.
Acquisition of data: Mihalopoulos, Dogra, Mohamed.
Analysis and interpretation of data: Mihalopoulos, Badani, Kyprianou.
Drafting of the manuscript: Mihalopoulos, Dogra, Mohamed, Badani.
Critical revision of the manuscript for important intellectual content: Kyprianou, Badani.
Statistical analysis: Mihalopoulos, Mohamed.
Obtaining funding: None.
Administrative, technical, or material support: Dogra, Mohamed.
Supervision: Kyprianou.
Other: None.
Financial disclosures: Natasha Kyprianou certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Malte Rieken
References
- 1.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. In press. 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed]
- 2.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) situation report—78. [Google Scholar]
- 4.Perico L., Benigni A., Remuzzi G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;24126:1–9. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323:1769–1770. doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B, Feng Z, Wang C, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. In press. 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed]
- 10.Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID-19 mortality and the renin-angiotensin system—a call for epidemiologic investigations. Clin Infect Dis. In press. 10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed]
- 11.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. In press. 10.1101/2020.01.31.929042. [DOI]
- 13.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anguiano L., Riera M., Pascual J., Soler M.J. Circulating ACE2 in cardiovascular and kidney diseases. Curr Med Chem. 2017;24:3231–3241. doi: 10.2174/0929867324666170414162841. [DOI] [PubMed] [Google Scholar]
- 16.de Farias Lelis D., de Freitas D.F., Machado A.S., Crespo T.S., Santos S.H.S. Angiotensin-(1-7), adipokines and inflammation. Metabolism. 2019;95:36–45. doi: 10.1016/j.metabol.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez L., Novoa U., Moya J. Angiotensin-(1-9) reduces cardiovascular and renal inflammation in experimental renin-independent hypertension. Biochem Pharmacol. 2018;156:357–370. doi: 10.1016/j.bcp.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Donoghue M., Hsieh F., Baronas E. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:e1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 19.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrario C.M., Jessup J., Chappell M.C. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 21.Qiao W., Wang C., Chen B. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131:97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak : a study based on single-cell transcriptome analysis. Intensive Care Med. In press. 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed]
- 24.10.1101/2020.02.08.20021212Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. MedRxiv. In press. . [DOI]
- 25.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda M., Martins R., Hendrie P.C. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. 2020;18:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 28.Board on Health Care Services Institute of Medicine . National Academies Press; Washington, DC: 2012. The role of telehealth in an evolving health care environment, workshop summary. [PubMed] [Google Scholar]
- 29.Wang Y., Zhou S., Yang F. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott B. U.S. coronavirus patients with other conditions hospitalized at higher rates. The Wall Street Journal. 2020 https://www.wsj.com/articles/u-s-coronavirus-patients-with-other-conditions-hospitalized-at-higher-rates-11585689057?mod=mhp [Google Scholar]
- 32.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Li X, Chen H, et al. SARS-CoV-2 infection does not significantly cause acute renal injury: an analysis of 116 hospitalized patients with COVID-19 in a single hospital, Wuhan, China. SSRN Electron J. In press. 10.2139/ssrn.3541116. [DOI]
- 34.Naicker S., Yang C.W., Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Extance A. Covid-19 and long term conditions: what if you have cancer, diabetes, or chronic kidney disease? BMJ. 2020;368:m1174. doi: 10.1136/bmj.m1174. [DOI] [PubMed] [Google Scholar]
- 36.Kliger A.S., Silberzweig J. Mitigating Risk of COVID-19 in Dialysis Facilities. Clin J Am Soc Nephrol. 2020;15:707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basile C., Combe C., Pizzarelli F. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35:737–741. doi: 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2006;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betjes M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 40.Jung J.Y., Park B.H., Hong S.B. Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: a multicenter study. J Crit Care. 2011;26:577–585. doi: 10.1016/j.jcrc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. In press. 10.1101/2020.03.04.20030395. [DOI]
- 42.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jefferson J.A., Nelson P.J., Najafian B., Shankland S.J. Podocyte disorders: core curriculum 2011. Am J Kidney Dis. 2011;58:666–677. doi: 10.1053/j.ajkd.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang K.J., Su I.J., Theron M. An interferon-γ-related cytokine storm in SARS patients. J Med Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capitanio U., Bensalah K., Bex A. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barry R.E., Krek W. The von Hippel-Lindau tumour suppressor: a multi-faceted inhibitor of tumourigenesis. Trends Mol Med. 2004;10:466–472. doi: 10.1016/j.molmed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Négrier S., Raymond E. Antiangiogenic treatments and mechanisms of action in renal cell carcinoma. Invest New Drugs. 2012;30:1791–1801. doi: 10.1007/s10637-011-9677-6. [DOI] [PubMed] [Google Scholar]
- 49.Aoun F., Rassy E.E., Assi T., Kattan J. PDL-1/PD1 inhibitors: antibody or antinobody? Future Oncol. 2017;13:1669–1671. doi: 10.2217/fon-2017-0215. [DOI] [PubMed] [Google Scholar]
- 50.Labriola M.K., Zhu J., Gupta R. Characterization of tumor mutation burden, PD-L1 and DNA repair genes to assess relationship to immune checkpoint inhibitors response in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escudier B., Porta C., Schmidinger M. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v58–68. doi: 10.1093/annonc/mdw328. [DOI] [PubMed] [Google Scholar]
- 52.Albiges L., Powles T., Staehler M. Updated European Association of Urology guidelines on renal cell carcinoma: immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol. 2019;76:151–156. doi: 10.1016/j.eururo.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Rassy E., Flippot R., Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol. 2018;12:1–13. doi: 10.1177/1758835920907504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobczuk P., Szczylik C., Porta C., Czarnecka A.M. Renin angiotensin system deregulation as renal cancer risk factor (Review) Oncol Lett. 2017;14:5059–5068. doi: 10.3892/ol.2017.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araújo W.F., Naves M.A., Ravanini J.N., Schor N., Teixeira V.P.C. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol Semin Orig Investig. 2015;33:389.e1–389.e7. doi: 10.1016/j.urolonc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 57.Dolley-Hitze T., Jouan F., Martin B. Angiotensin-2 receptors (AT1-R and AT2-R), new prognostic factors for renal clear-cell carcinoma. Br J Cancer. 2010;103:1698–1705. doi: 10.1038/sj.bjc.6605866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larrinaga G., Pérez I., Sanz B. Angiotensin-converting enzymes (ACE and ACE2) are downregulated in renal tumors. Regul Pept. 2010;165:218–223. doi: 10.1016/j.regpep.2010.07.170. [DOI] [PubMed] [Google Scholar]
- 59.Ni L., Feng Y., Wan H. Angiotensin-(1-7) inhibits the migration and invasion of A549 human lung adenocarcinoma cells through inactivation of the PI3K/Akt and MAPK signaling pathways. Oncol Rep. 2012;27:783–790. doi: 10.3892/or.2011.1554. [DOI] [PubMed] [Google Scholar]
- 60.Soto-Pantoja D.R., Menon J., Gallagher P.E., Tallant E.A. Angiotensin-(1-7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Mol Cancer Ther. 2009;8:1676–1683. doi: 10.1158/1535-7163.MCT-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallagher P.E., Tallant E.A. Inhibition of human lung cancer cell growth by angiotensin-(1-7) Carcinogenesis. 2004;25:2045–2052. doi: 10.1093/carcin/bgh236. [DOI] [PubMed] [Google Scholar]
- 62.Enevoldsen F.C., Sahana J., Wehland M., Grimm D., Infanger M., Krüger M. Endothelin receptor antagonists: status quo and future perspectives for targeted therapy. J Clin Med. 2020;9:824. doi: 10.3390/jcm9030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Houde M., Desbiens L., D’Orléans-Juste P. Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol. 2016;77:143–175. doi: 10.1016/bs.apha.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Diaz L.A., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith J.T., Wunsch B.H., Dogra N. Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip. 2018;18:3913–3925. doi: 10.1039/c8lc01017j. [DOI] [PubMed] [Google Scholar]
- 66.Stott S.L., Hsu C.H., Tsukrov D.I. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murillo O., Thistlethwaite W., Rozowsky J. ExRNA Atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell. 2019;177:463–477. doi: 10.1016/j.cell.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Navneet D., Xuelian L., Kohli P. Investigating ligand−receptor interactions at bilayer surface using electronic absorption spectroscopy and fluorescence resonance energy transfer. Langmuir. 2012;28:12989–12998. doi: 10.1021/la300724z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Redova M., Poprach A., Nekvindova J. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med. 2012;10(1):55. doi: 10.1186/1479-5876-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karachaliou N., Mayo-de-las-Casas C., Molina-Vila M.A., Rosell R. Real-time liquid biopsies become a reality in cancer treatment. Ann Transl Med. 2015;3:2–4. doi: 10.3978/j.issn.2305-5839.2015.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raimondo F., Morosi L., Corbetta S. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol Biosyst. 2013;9:1220–1233. doi: 10.1039/c3mb25582d. [DOI] [PubMed] [Google Scholar]
- 72.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arabi Y.M., Arifi A.A., Balky H.H. Clinical course and outcomes of critically Ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 74.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park B.K., Maharjan S., Lee S.I. Generation and characterization of a monoclonal antibody against MERS-CoV targeting the spike protein using a synthetic peptide epitope-CpG-DNA-liposome complex. BMB Rep. 2019;52:397–402. doi: 10.5483/BMBRep.2019.52.6.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chu C.M., Cheng V.C.C., Hung I.F.N. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]