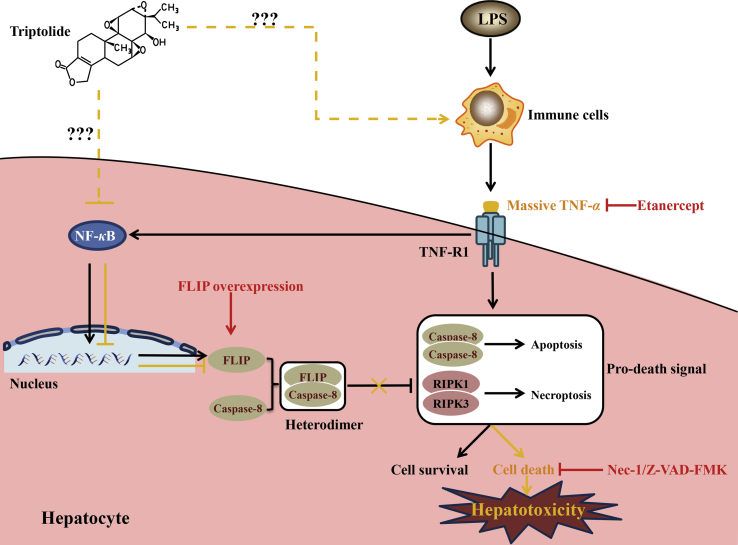
Figure 8.
Schematic presentation indicated the suggested mechanisms by which TP/LPS-induced hepatotoxicity and the possible mechanistic intervention of etanercept, Z-VAD-FMK, Nec-1, as well as FLIP overexpression for liver protection in TP/LPS-induced liver injury. Under physiological conditions, the binding of LPS on the surface of TLR4 on immune cells triggers the production of TNF-α. On one hand, the attachment of TNF-α to TNF-R1 on hepatocytes facilitated the formation of cell death Complex IIa and Complex IIb, thus promoting TNF-α treated hepatic cell death. On the other hand, stimulation of TNF-R1 activated NF-κB and up-regulated the expression of pro-survival proteins (including FLIP) to inhibit cell death. Thus, TNF-α alone cannot induce hepatic cell death due to the balance between pro-survival and pro-death signals (indicated as black arrows). However, TP treatment inhibited the NF-κB-dependent transcriptional activity and the subsequent pro-survival protein FLIP in hepatocytes. TP-treated hepatocytes cannot counteract TNF-α induced cell death, ultimately promoting hepatic cell death in the presence of TNF-α (indicated as orange arrows). Z-VAD-FMK and Nec-1 inactivated apoptosis and necroptosis, thus alleviated TP/LPS-induced hepatotoxicity. Etanercept treatment inhibited the binding of TNF-α to TNF-R1 and protected mice from TP/LPS-induced hepatotoxicity. FLIP overexpression increased the formulation of FLIP–caspase-8 complex, ultimately inhibited TP/LPS-induced cell death and hepatotoxicity.