Figure 4.
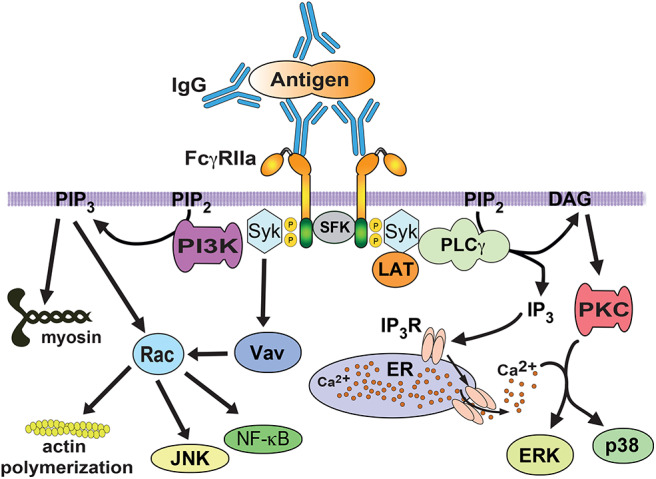
FcγR signaling for phagocytosis. FcγRIIa crosslinking by immunoglobulin (IgG) bound to a particle, induces activation of Src family kinases (SFK), which phosphorylate tyrosine residues in the ITAM sequence within the cytoplasmic tail of the receptor. Then, spleen tyrosine kinase (Syk) associates with phosphorylated ITAMs and leads to phosphorylation and activation of a signaling complex formed by the scaffold protein LAT (linker for activation of T cells) interacting with various proteins. One of these proteins is phospholipase C gamma (PLCγ), which produces inositoltrisphosphate (IP3), and diacylglycerol (DAG). These second messengers cause calcium release and activation of protein kinase C (PKC), respectively. PKC leads to activation of extracellular signal-regulated kinases (ERK and p38). The guanine nucleotide exchange factor Vav activates the GTPase Rac, which is involved in regulation of the actin cytoskeleton. Rac is also involved in activation of transcription factors such as NF-κB and JNK. The enzyme phosphatidylinositol 3-kinase (PI3K), which is recruited and activated by Syk, generates the lipid phosphatidylinositol-3,4,5-trisphosphate (PIP3) at the phagocytic cup. This lipid also regulates Rac activation, and contractile proteins such as myosin. P represents a phosphate group. ER, endoplasmic reticulum. IP3R, receptor (calcium channel) for inositoltrisphosphate.
