Figure 3.
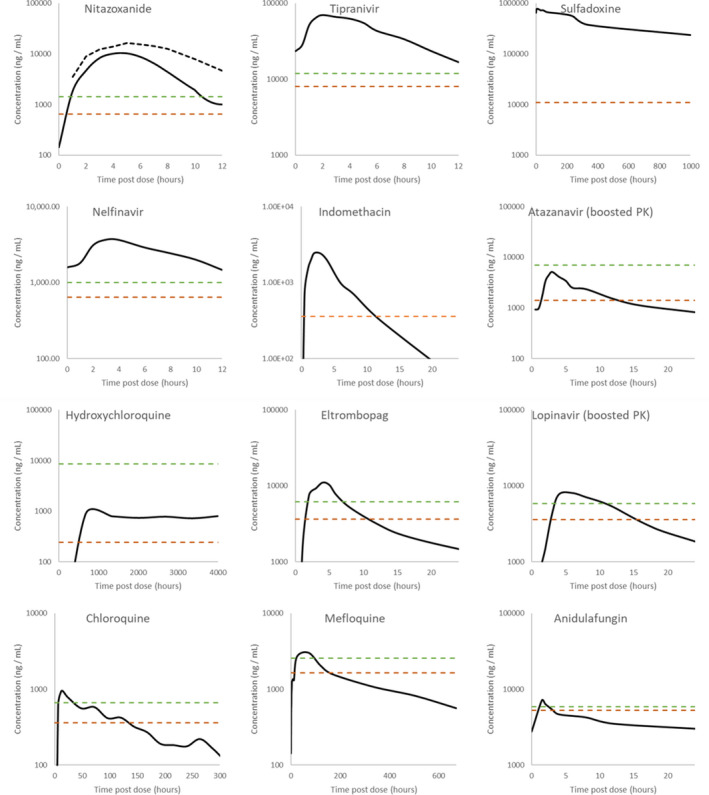
Digitized pharmacokinetic (PK) interrogation of all drugs calculated to have a peak plasma concentration (Cmax)/half‐maximal effective concentration (EC50) ratio above 1. The lowest reported severe acute respiratory syndrome‐coronavirus 2 EC50 (dashed orange lines) and associated recalculated effective concentration 90% (EC90; dashed green lines) are also highlighted. References for the utilized data are nitazoxanide 500 mg b.i.d. and 1,000 mg b.i.d., 95 tipranavir 500 mg b.i.d. with 200 mg ritonavir, 96 sulfadoxine 1,500 mg with 75 mg pyrimethamine, 82 nelfinavir 1,250 mg b.i.d., 97 indomethacin 50 mg t.i.d., 98 atazanavir 300 mg q.d. with 100 mg ritonavir, 99 hydroxychloroquine 2,000 mg hydroxychloroquine sulfate/1,550 mg base administered over 3 days, 100 eltrombopag 75 mg single dose, 101 lopinavir 400 mg with 100 mg ritonavir, 102 chloroquine 1,500 mg administered over 3 days, 103 mefloquine 1,200 mg over 3 days, 104 and anidulafungin 100 mg q.d. 105 Robust PK data were unavailable for niclosamide 500 mg, ritonavir 600 mg, and merimepodib 300 mg in order to conduct this digitized interrogation of these molecules.
