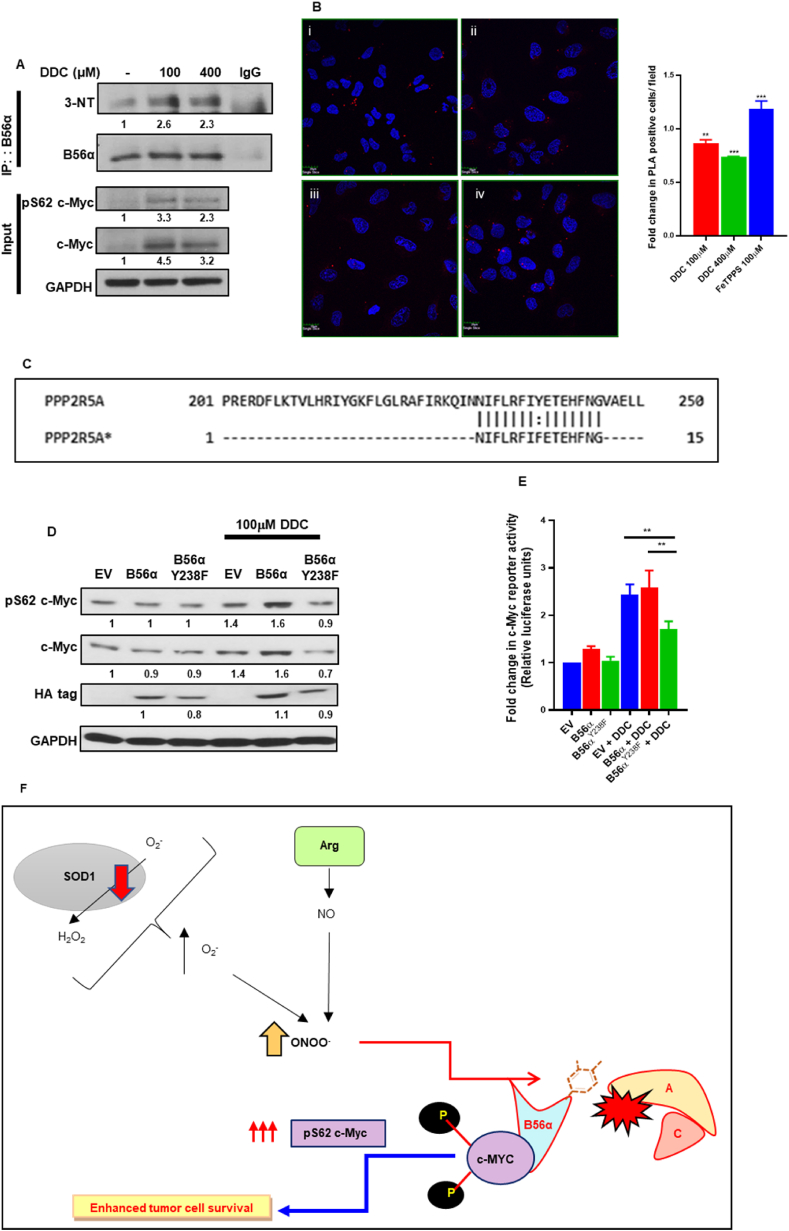
Fig. 7.
Peroxynitrite disrupts PP2A holoenzyme assembly by nitration of B56α at the Y238 position: (A) Immunoprecipitation of B56α demonstrated increased 3-nitrotyrosine mark in the presence of DDC treatment in PANC-1 cells. (B) PLA assay demonstrates decreased interaction between scaffold subunit PP2A-A and B56α when U2-OS cells were subjected to DDC treatments (4 h), whereas treatment with FeTPPS (6 h) demonstrates a reciprocal increase in interaction (i – Control, ii – DDC 100 μM, iii – DDC 400 μM, iv – FeTPPS 100 μM). (C) Primer design for site-directed mutagenesis of B56α Y238 to non-nitratable phenylalanine. (D) Western blot analyses of pS62 c-Myc and total c-Myc protein levels in EV, B56α and B56αY238F mutant cells in the presence of DDC (100 μM/4 h). (E) c-Myc reporter luciferase activity assay analyses of EV, HA tagged B56α and HA tagged B56αY238F mutant cells subjected to DDC (100 μM/4 h) treatment. Fold change in c-Myc reporter activity was normalized to EV control. (F) Summary graphical model of pro-oxidant redox dependent stabilization and activation of c-Myc oncoprotein in cancer cells – Mild increase in superoxide levels is followed by a rise in intracellular peroxynitrite levels, which leads to nitrative inhibition of B56α at the Y238 position, subsequently resulting in the sustained phospho-stabilization and activation of c-Myc, which drives the oncogenic programs in the tumor cells. One-way ANOVA was employed for statistical analysis (** = p < 0.01, *** = p < 0.001).