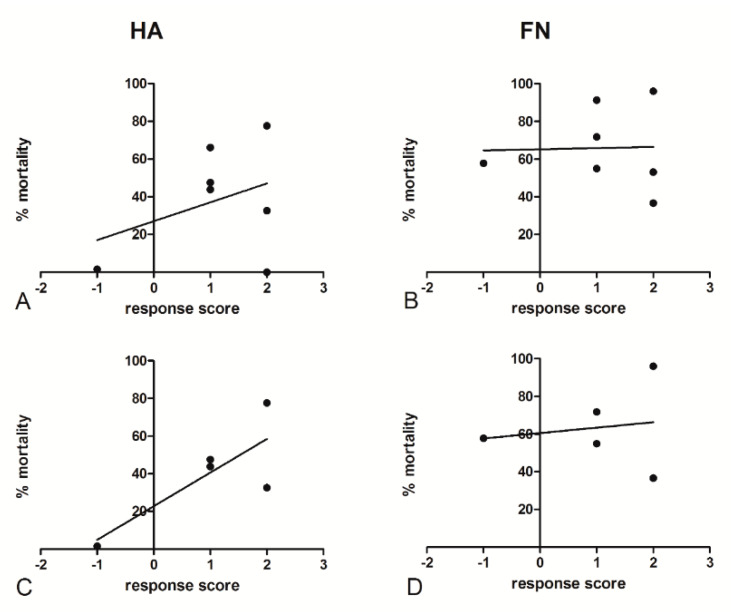
Figure 9.
Analysis of the relationship between primary cells’ in vitro mortality and patients’ responses to chemotherapy. RECIST guideline parameters were associated to a response score after the first three cycles of cisplatin treatment: −2/−1, progressive disease (PD); 0, stable disease (SD); +1, partial response (PR); and +2, complete response (CR). No significant correlation was identified for the killing assays performed on the FN matrix (B), whereas those on HA showed a moderate correlation coefficient (A); the correlation coefficient was higher when BRCA-mutated patients were excluded (C). No significant correlation was identified for the killing assays performed on the FN matrix even when BRCA-mutated patients were excluded (D). Pearson’s test allowed us to perform a correlation assessment by taking into account the percentage of in vitro mortality after 5 μg/mL cisplatin treatment, both on HA and FN, and the clinical response score.