Figure 1.
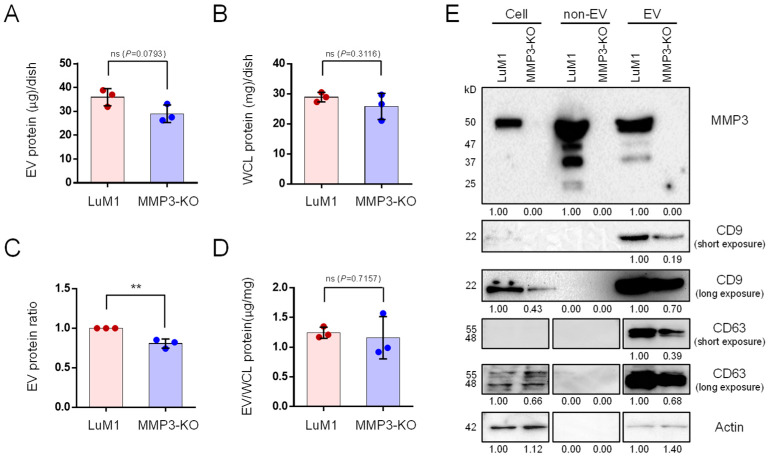
MMP3 knockout reduces the release of CD9 and CD63 within extracellular vesicles. Tumoroids were formed in 10-cm ultra-low attachment (ULA) plates for 6 days. Extracellular vesicle (EV) and non-EV fractions were collected from the culture supernatants. (A,B) The total protein concentration in the (A) EV and (B) whole cell lysate (WCL) fractions of LuM1-tumoroids and MMP3-KO tumoroids. (C) Relative EV protein ratio comparing two cell lines. (D) EV protein concentration per the WCL proteins. ** p < 0.01; ns, not significant. (E) Western blotting showing MMP3, CD9, CD63, and β-actin in tumoroids, non-EV, and EV fractions. The 54-kD bands indicate the full-length MMP3, the 47-kD bands represent the active form consists of the catalytic, hinge, and PEX domains, the 37-kD represents the catalytic domain, and the 25-kD shows the PEX domain of MMP3. The expression level of β-actin was examined as a loading control as well as to check if it was released from cells. The protein amounts loaded from WCL and EV fractions were 10 µg per lane, while 5 µg per lane were loaded from the non-EV fractions. The experiments were repeated twice. For full images of Western blotting, see Figure S1.