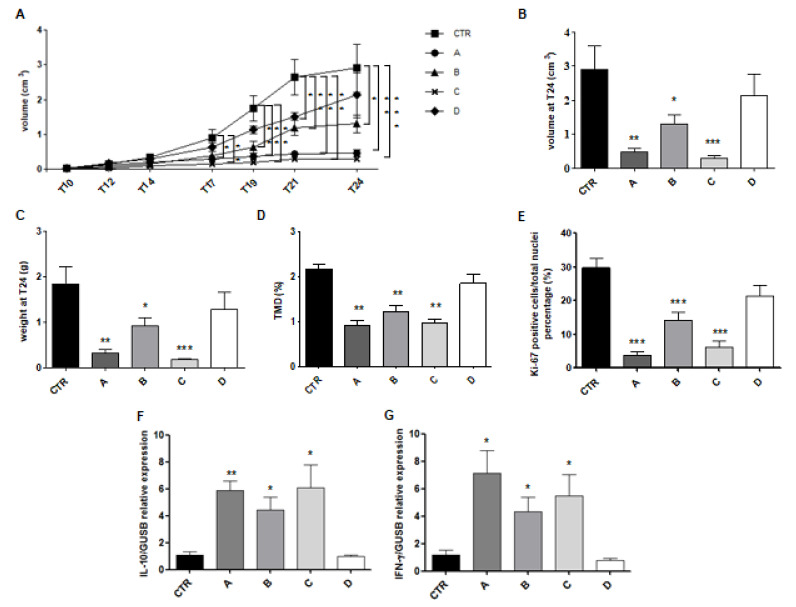
Figure 6.
Results of animal experiments. Statistical analysis: (A) Tumour volume: formulations vs. CTR * p < 0.05; ** p < 0.01; *** p < 0.005; (B) Tumour volume at endpoint: CTR vs. B * p < 0.05; CTR vs. A ** p < 0.01; CTR vs. C *** p < 0.005; (C) Tumour mass at endpoint: CTR vs. B * p <0.05; CTR vs. A ** p < 0.01; CTR vs. C *** p < 0.005; (D) CD31 quantification: CTR vs. A, B, C ** p < 0.01; (E) Ki67 quantification: CTR vs. A, B, C *** p < 0.005; (F) IL-10 quantification: CTR vs. A ** p < 0.01; CTR vs. B, C * p < 0.05; (G) IFN-γ quantification: CTR vs. A, B, C * p < 0.05. CTR: control; A: Intralipid® (IL) loaded with combination of drugs (MIX) and high dose of rapamycin (RAP); B: IL MIX-RAP low dose; C: MIX-RAP high dose; D: MIX-RAP low dose.