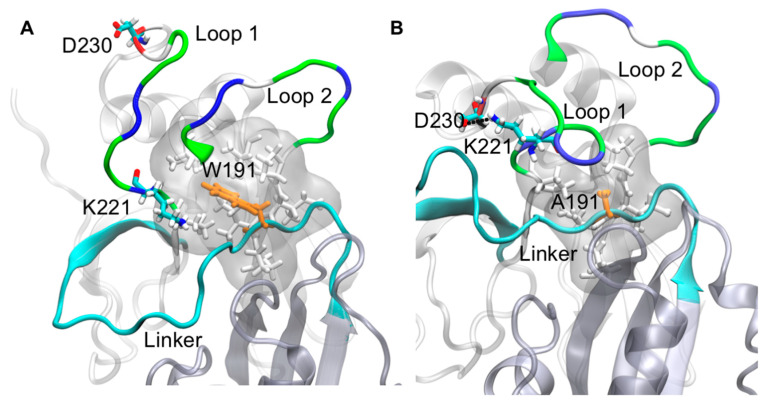
Figure 5.
Molecular dynamics (MD) simulations of WT VP40 and W191A C-terminal domain flexibility. (A) MD simulations indicate that WT VP40 has a flexible lipid-binding Loop 1 (containing lipid-binding residues Lys224 and Lys225) and a fairly restricted lipid-binding Loop 2 (lipid binding residues Lys274 and Lys275) (See also Supplementary Materials Video S1). (B) MD simulations with W191A demonstrate a significant change in Loop 1 flexibility due to a K221–D230 salt-bridge, a similar level of flexibility of Loop 2 (albeit a slightly different orientation), and a more flexible linker between the N-terminal domain (NTD) and CTD than that of WT.