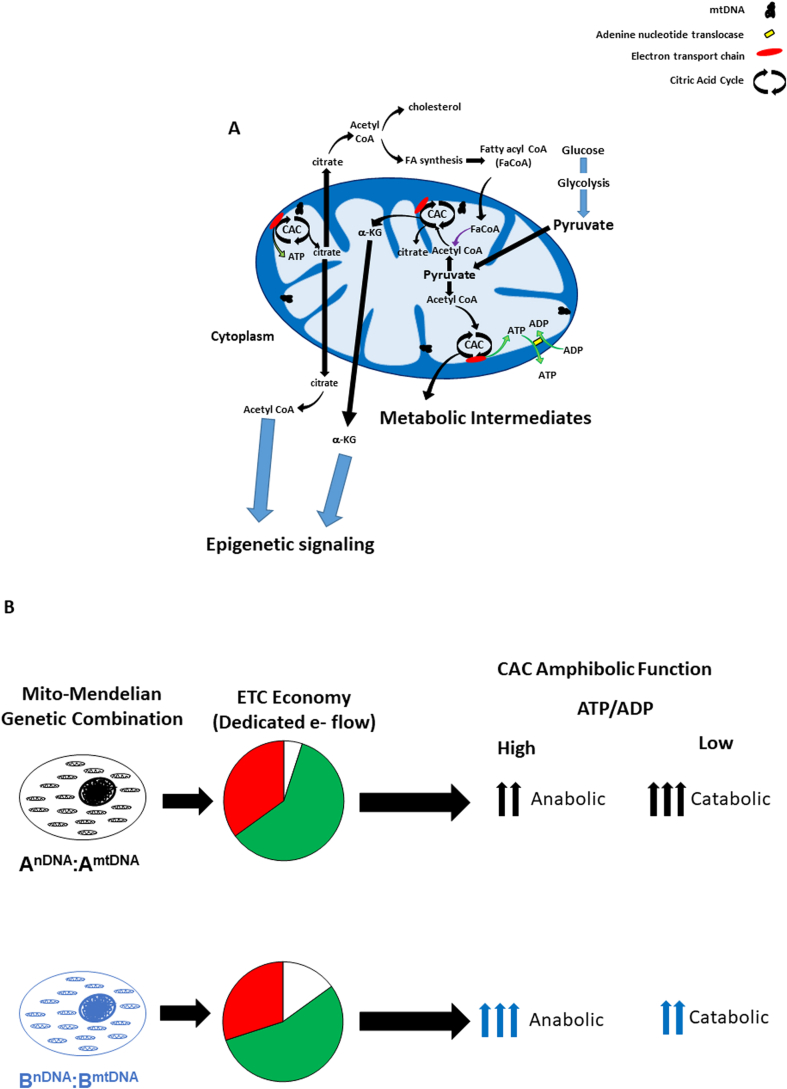
Fig. 3.
Mitochondrial citric acid cycle (CAC) metabolism provides fuel for multiple cellular processes (see text for greater detail). A) Glucose, glucogenic amino acid carbon skeletons and triglycerides can be metabolized to pyruvate which is oxidatively decarboxylated to form acetyl-CoA. Mitochondrial ATP is translocated out of the mitochondrion with exchange of ADP by the adenine nucleotide translocase (ANT). During lipogenesis, citrate is shuttled out of the mitochondrion into the cytosol and cleaved to yield acetyl-CoA that can utilized for fatty acid or cholesterol synthesis. In turn, fatty acid oxidation in the mitochondrion (fatty acyl CoA, FaCoA) generates acetyl CoA from fatty acids. Multiple CAC metabolic intermediates can be used for a variety of cellular biosynthetic pathways, including citrate and α-ketoglutarate, which play important roles in epigenetic processes (see text, Epigenetic Signaling). B) The mito-Mendelian paradigm hypothesizes that genetic mutations which convey changes in mitochondrial economy (e.g. dedicated e- flow for ATP production) impact the amphibolic balance of the CAC. For example, the bioenergetic profile from the AnDNA:AmtDNA combination shows greater dedicated e- flow for ATP production relative to the BnDNA:BmtDNA combination (pie charts). Hence, as ATP/ADP ratios increase, the demand for CAC generated reducing equivalents for energy production (oxidative phosphorylation) declines, and CAC flux shifts towards a more anabolic balance – yet because the AnDNA:AmtDNA combination utilizes more e- flow for ATP production compared to the BnDNA:BmtDNA combination, less metabolic intermediates are available for anabolic processes relative to the BnDNA:BmtDNA combination. Conversely, as ATP/ADP ratios decrease, the AnDNA:AmtDNA combination exhibits higher level of catabolism relative to the BnDNA:BmtDNA combination to meet energetic needs due to its lower mitochondrial economy.