1. Introduction
Functional pain syndromes (FPS), including fibromyalgia (FM), temporomandibular disorder (TMD), tension-type headache (TTH), irritable bowel syndrome (IBS), and low back pain (LBP) represent a significant healthcare problem that affect nearly one in three Americans, predominantly females.[18; 25; 73; 77; 89; 102] These conditions are characterized by persistent pain in the absence of tissue damage and often co-occur, thereby affecting multiple body sites. Accumulating evidence suggests that the origins of FPS are linked to abnormalities in pathways that regulate catecholamine bioavailability. Patients with FPS have increased circulating basal and stress-induced levels of the catecholamines epinephrine and norepinephrine [9; 27; 70; 87], and reduced activity of catechol-O-methyltransferase (COMT),[60; 80] a ubiquitously expressed enzyme that metabolizes catecholamines.[59] An estimated 66% of FPS patients have variants in the COMT gene that result in lower COMT activity.[49; 53; 66; 82; 94] The ‘low COMT activity’ variants are associated with the onset of FM[5; 20; 34; 62; 92] and TMD[24], increased pain in response to experimental stimuli,[24; 113] and pain-related comorbidities such as depression.[6; 28; 41; 49; 52; 76; 94]
In previous pre-clinical studies, we demonstrated that genetic knockout or pharmacologic inhibition of COMT results in pain at multiple body sites that persists for months, pain-related volitional behaviors (eg, avoidance of bright light), and systemic inflammation via activation of adrenergic receptor β2 and β3.[17; 46; 47; 111] This animal model of COMT-dependent functional pain, defined as increased pain following COMT inhibition, has provided valuable mechanistic insight into how genetically-driven increases in basal catecholamine levels lead to chronic functional pain. However, it is important to consider how the effects of the low COMT activity genetic predisposition are modified by environmental events, such as stress. Stress activates the sympathetic nervous system, stimulating the release of catecholamines so as to induce pain in healthy individuals while exacerbating pain in those with FPS.[61] In fact, individuals with the low COMT activity genotype report increased pain after stressful events, such as a motor vehicle accident.[64; 78] The independent and joint contributions of low COMT and stress to FPS and related comorbidities such as depression, which also predominantly affect females, have remained unclear.
Thus, the present study sought to examine the contributions of low COMT and/or stress to functional pain and depressive-like behavior, as well as molecular correlates of these behaviors. We hypothesized that stress would exacerbate the degree of COMT-dependent functional pain and that COMT inhibition would exacerbate stress-dependent depressive-like behavior. Further, we hypothesized that low COMT and stress would differentially affect the expression of brain-derived neurotrophic factor (BDNF; a factor involved in nociception and depression) and glucocorticoid receptor (GR; a stress-related receptor) in the spinal cord and hippocampus, in a sex-dependent manner. To test these hypotheses, separate groups of mice receiving sustained delivery of the COMT inhibitor OR486 or vehicle were subjected to repeated forced swim stress or sham swim. Pain behavior and depressive-like behavior were measured over the course of 14 days. BDNF and GR expression in the spinal cord and hippocampus CA1 area was measured on day 14 using immunohistofluorescence and Western blot.
2. Materials and Methods
2.1. Animals
Adult 8–12 week-old male (N=56) and female (N=62) C57BL/6 mice weighing 18–30g were bred in-house. Mice had ad libitum access to standard laboratory chow and water, and were maintained under a 10 hr light / 14 hr dark cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee at Duke University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. General experimental design
Here, we aimed to determine the independent and joint effects of low COMT and stress on pain and comorbid depressive-like behavior, as well as molecular correlates of these behaviors in male and female mice. Male and female mice were randomly assigned to one of four experimental groups; 1) OR486 + swim sham (OR486+Sham), 2) OR486 + swim stress (OR486+Stress), 3) Vehicle + swim sham (Vehicle+Sham), and 4) Vehicle + swim stress (Vehicle+Stress). The COMT inhibitor OR486 or vehicle was delivered over 14 days, and mice were subjected to the forced swim or sham paradigm on days 8–10 during OR486 or vehicle delivery.
Mice were handled and habituated for 4 days prior to minipump implantation. Pain behaviors evoked by mechanical and thermal stimuli were evaluated at baseline (on day 0), prior to swim stress or swim sham (on days 6–7), during swim stress or swim sham (on days 9–10), and following swim stress or swim sham (on days 12–13). Depressive-like behavior during the tail suspension test was evaluated on day 13. On day 14, mice were euthanized and spinal cord and hippocampal tissues were collected to measure the expression of BDNF and GR. Experimenters performing pain assessments and quantifying BDNF and GR expression were blinded to both the drug and stress conditions.
2.3. COMT-dependent functional pain
We modeled COMT-dependent functional pain in mice as described previously.[17; 46; 111] In brief, Alzet osmotic minipumps (model 1002, Durect Corporation, Cupertino, CA, USA) were implanted subcutaneously in the intrascapular region for systemic delivery of vehicle or the COMT inhibitor OR486 (Tocris Bioscience, Bristol, UK) over 14 days at a dose sufficient to cross the blood brain barrier.[67] The OR486 was dissolved in a 5:2:3 ratio mixture of dimethyl sulfoxide: ethanol: 0.9% sterile saline and injected into the pump which was subsequently incubated overnight at 37°C in a 15mL conical tube containing sterile saline.
2.4. Forced swim stress
As described previously,[13] mice were subjected to the forced swim or sham stress paradigm on days 8–10 (10 min on day 8, 20 min on days 9 and 10). Mice were placed in their respective glass swimming chambers (45.72 cm in height × 20.23 cm in diameter) that contained 20 cm (swim stress) or 2 cm (swim sham) of water maintained at 24–26°C. Following each swim session, mice were dried with a towel and provided with a heat source until dry.
2.5. Pain behavior testing
Paw withdrawal threshold to punctate mechanical stimuli was assessed using the von Frey up–down method. A series of nine von Frey monofilaments with logarithmically increasing stiffness (0.008–1.5 g, Stoelting, Wood Dale, IL, USA) was presented perpendicularly to the central plantar surface of the hindpaw. First, the middle filament (#5, 0.17 g) was applied to the hindpaw for 3 s. If the mouse responded with a withdrawal, an incrementally lower filament was applied. In the absence of a withdrawal, an incrementally higher filament was applied. A series of six total responses were recorded for each paw. Results were determined using a logarithmic algorithm to determine the gram-force value that would elicit paw withdrawal in 50% of trials (10(Xf + kδ)/10,000, where Xf = value [in log units] of the final von Frey filament used; k = tabular value of positive and negative responses, and δ = mean difference [in log units] between stimuli). Mechanical allodynia was defined as a significant decrease in paw withdrawal threshold from baseline.
After determining paw withdrawal threshold, paw withdrawal frequency to a noxious von Frey monofilament was assessed. A higher gram force filament (#7, 0.7 g) was applied to the hind paw 10 times for a duration of 1 s, with an inter-stimulus interval of 1 s. The number of paw withdrawals (ranging from 0–10) was recorded for each hindpaw. Mechanical hyperalgesia was defined as a significant increase in the number of paw withdrawals from baseline.
Finally, paw withdrawal latency to thermal heat was assessed using the Hargreaves method.[36] Mice were placed in plexiglass chambers, and a radiant beam of light was applied to the hindpaw through a glass floor. Paw withdrawal latencies were recorded in duplicate per paw. If the second latency recorded was not within ± 4seconds of the first, a third measure was recorded. The two latencies closest in value were averaged to determine overall latency to withdrawal. Thermal hyperalgesia was defined as a decrease in paw withdrawal latency from baseline.
2.6. Depressive-like behavior
Depressive-like behavior was assessed using the tail suspension test (TST).[14] In brief, mice were suspended for 6 minutes from their tails, taped to a table 20–25 cm from the floor, such that they could not escape or hold onto nearby surfaces. Immobility time, which serves as an indicator of despair/depression, was recorded and quantified. Immobility time was also recorded during the forced swim stress sessions as previously described.[106]
2.7. Immunohistochemical staining
On day 14, mice were deeply anesthetized with an intraperitoneal injection of 300mg/kg Fatal Plus (Vortech Pharmaceuticals, Dearborn, MI, USA), and subsequently perfused intracardially with 50 mL of ice-cold heparinized 0.1 M phosphate-buffered saline. This was followed by 25–50 ml fixative (4% paraformaldehyde in 0.1 M sodium phosphate buffer) applied at a rate of 20 ml/min. The lumbar-sacral segment of the spinal cord and the hippocampus were isolated, post-fixed in 4% paraformaldehyde for 12 hr, and finally immersed in a 30% sucrose solution for storage at 4°C until sectioning. Transverse sections were cut to a thickness of 30 μm using a cryostat (HM 550, Microm International GmbH, Walldorf, Germany), incubated in 5% fetal bovine blocking serum in 0.01 M phosphate-buffered saline at room temperature for 1 hr, and stained overnight at 4°C with the rabbit anti-BDNF primary antibody (ab108319, 1:500; Abcam, Cambridge, United Kingdom), the rabbit anti-GR primary antibody (1:500; Thermo Scientific, Waltham, MA, USA), or with the mouse anti-NeuN antibody (1:500; Millipore Sigma, St. Louis, MO, USA). The following day, the sections were incubated with the anti-rabbit Alexa-594 IgG (1:500; Jackson ImmunoResearch Inc., West Grove, PA, USA) and anti-mouse Alexa-488 IgG (1:500; Jackson ImmunoResearch Inc., USA) secondary antibodies for 1 hr at room temperature. The sections were then mounted on gelatin-coated slides (VWR, Radnor, PA, USA), allowed to air-dry until complete dehydration, and coverslipped. The specificity of immunohistochemical staining was verified by omission of the primary antibody or omission of the secondary antibody from the staining protocol for each set of experiments.
Histological features of the spinal dorsal horn and hippocampus CA1 area were captured using a Zeiss 780 upright confocal microscope (Oberkochen, Germany). Expression of BDNF and GR were quantified by optical density using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.8. Western blot
Flash frozen spinal cord tissue was harvested on day 14 from a separate cohort of mice to confirm BDNF and GR antibody specificity. The tissue was homogenized in a Precellys 24 tissue homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France) with tissue protein extraction reagent (TPER, ThermoFisher). To accommodate for variation in tissue homogenates, protein concentrations were normalized following bicinchoninic acid (BCA; Pierce, Grans Island, NY, USA) measurement of protein content to evenly load protein into wells of 4–20% SDS-PAGE gels (BioRad). Gels were run using standard SDS-PAGE methods and transferred onto PVDF membranes using the iBlot2 dry blotting system (Life Technologies). Membranes were then blocked, probed with primary antibodies for GAPDH (1:1000; Cell Signaling, Danvers, MA, USA), BDNF (ab108319, 1:1000; Abcam, Cambridge, United Kingdom) or GR (1:1500; Thermo Scientific, Waltham, MA, USA), and subsequently probed with a corresponding secondary antibody. Specific bands were visualized with enhanced chemiluminescence (Thermo Scientific, Waltham, MA, USA) and quantified with Image J. The relative levels of BDNF and GR were normalized to GAPDH.
2.9. Statistical analyses
Group differences in behavioral responses and immunohistochemical fluorescent intensities were analyzed by 1-way or 2-way ANOVA. Post hoc comparisons were performed using the Bonferroni test, which corrected for multiple comparisons. Statistical significance was defined as P < 0.05. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA).
2. Results
3.1. Effect of Stress on COMT-dependent Functional Pain in Males and Females
Here, we sought to examine the impact of COMT- and stress-dependent increases in catecholaminergic tone on chronic pain and depressive-like behavior as well as molecular correlates of these behaviors. First, we examined the independent and joint contributions of low COMT and stress to pain. In line with our previous work, we found that sustained systemic delivery of the COMT inhibitor OR486 produced pronounced increases in pain behavior in both males and females (Figure 1).[46; 111] Compared to males and females in the Vehicle+Sham group, those in the OR486+Sham group exhibited mechanical allodynia (Two-way ANOVA for group × time. Males: F3,76 = 8.61, P < 0.001 (Fig 1A) and females: F3,97 = 5.07, P < 0.001 (Fig 1B)), mechanical hyperalgesia (Two-way ANOVA for group × time. Males: F3,76 = 18.47, P < 0.001 (Fig 1C) and females: F3,97 = 15.18, P < 0.001 (Fig 1D)), and thermal heat hyperalgesia (Two-way ANOVA for group × time. Males: F3,76 = 29.44, P < 0.001 (Fig 1E) and females: F3,97 = 18.89, P < 0.001 (Fig 1F)) over the 14-day time course. The degree of COMT-dependent pain did not differ between males and females in the OR486+Sham group (Figure 1s).
Figure 1. Stress exacerbates COMT-dependent functional pain, especially among females.
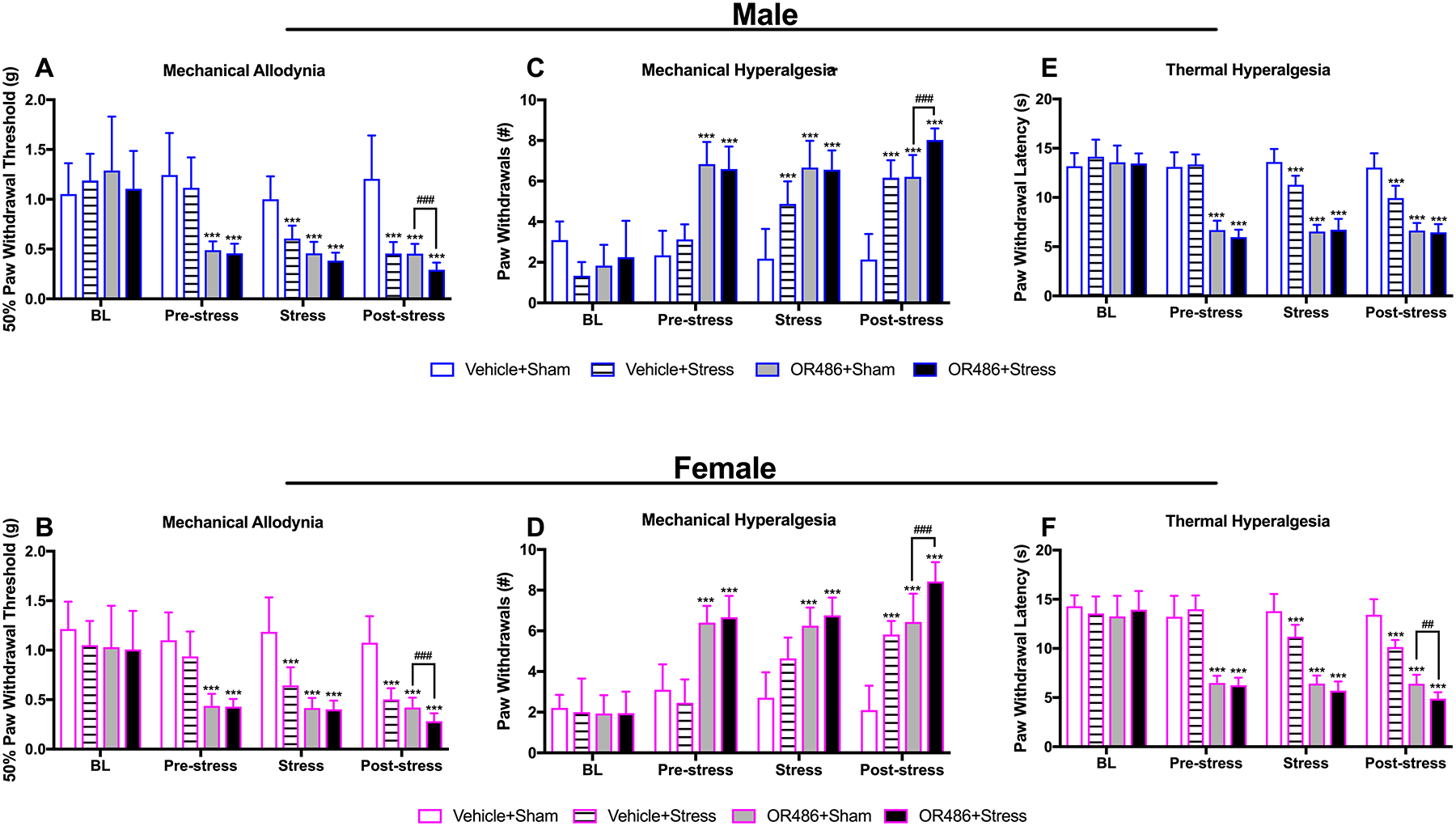
Sustained systemic delivery of the COMT inhibitor OR486 or 3 days of swim stress each produce pronounced (A, B) mechanical allodynia, (C, D) mechanical hyperalgesia, and (E, F) thermal heat hyperalgesia in both males and females. OR486 and swim stress together lead to significantly greater mechanical allodynia and mechanical hyperalgesia in males and females and greater thermal hyperalgesia only in females. Data are shown as Mean ± SD, N=6–9 per group. ***P < 0.001 different from the Vehicle+Sham group; ##P < 0.01, ###P < 0.001 different from the OR486+Sham group.
Similarly, we found that a 3-day repeated swim stress on days 8–10 produced moderate increases in pain behavior on days 12–13 post-stress in both males and females (Figure 1). Compared to males and females in the Vehicle+Sham group, those in the Vehicle+Stress group exhibited mechanical allodynia (Two-way ANOVA for group × time. Males: F3,76 = 7.335, P < 0.001 (Fig 1A) and females: F3,90 = 4.84, P < 0.005 (Fig 1B)), mechanical hyperalgesia (Two-way ANOVA for group × time. Males: F3,76 = 21.76, P < 0.001 (Fig 1C) and females: F3,90 = 20.34, P < 0.001 (Fig 1D)), and thermal heat hyperalgesia (Two-way ANOVA for group × time. Males: F3,76 = 10.98, P < 0.001, (Fig 1E) and females: F3,90 = 9.8, P < 0.001 (Fig 1F)). The degree of stress-dependent pain did not differ between males and females in the Vehicle+Stress group (Figure 1s).
Next, we found that sustained systemic OR486 delivery together with repeated swim stress produced significantly greater pain on days 12–13 post-stress than either OR486 delivery or swim stress alone (Figure 1). Compared to males and females in the OR486+Sham and Vehicle+Stress groups, those in the OR486+Stress group exhibited significant increases in mechanical allodynia (Two-way ANOVA for group × time. Males: F2,37 = 14.21, P < 0.001 (Fig 1A) and females: F2,48 = 23.42, P < 0.001 (Fig 1B), and mechanical hyperalgesia (Two-way ANOVA for group × time. Males: F2,37 = 23.56, P < 0.001 (Fig 1C) and females: F2,48 = 30.39, P < 0.001 (Fig 1D)). Of note, females in the OR486+Stress group exhibited greater thermal heat hyperalgesia compared to those in the OR486+Sham and Vehicle+Stress groups (Two-way ANOVA for group × time. F2,48 = 199.7, P < 0.001 (Fig 1F)), while males in the OR486+Stress group only exhibited greater thermal heat hyperalgesia compared to those in the Vehicle+Stress group, but not the OR486+Sham group (Two-way ANOVA for group × time. F2,37 = 52.32, P < 0.001 (Fig 1E)). A direct comparison of pain behavioral responses post-stress revealed a sex difference, such that thermal heat hyperalgesia was exacerbated in females. Thus, stress exacerbates COMT-dependent functional pain, especially among females.
3.2. Effect of Stress on COMT-dependent increases in Spinal BDNF Expression in Males and Females
Spinal BDNF increases the net excitatory drive of nociceptive neurons, and has been shown to play a key role in the onset and persistence of numerous chronic inflammatory and neuropathic pain conditions.[23; 84] Here, for the first time, we evaluated spinal BDNF expression in our mouse model of functional pain in the presence and absence of stress. We found that sustained systemic delivery of OR486 led to increases in spinal BDNF expression in both males and females, and swim stress potentiated this increase in females, but not males (Figure 2). Compared to the Vehicle+Sham group, male and female mice in the OR486+Sham group exhibited increased BDNF expression in lamina I-VI of the spinal dorsal horn (Two-way ANOVA for group × lamina. Males: F1,74 = 40.17, P < 0.001 (Fig 2A,C) and females: F1,74 = 22.8, P < 0.001 (Fig 2B,D)). Compared to their respective OR486+Sham groups, females, but not males, in the OR486+Stress group exhibited significantly higher increases in spinal BDNF expression (Two-way ANOVA for group × lamina. F1,74 = 32.27, P < 0.001 (Figure 2B,D)). Analysis of between-subjects factors in the same ANOVA revealed that the potentiation of stress-dependent increases in spinal BDNF expression by OR486 in female vs male mice is significant (One-way ANOVA for group. F5,42 = 11.79, P < 0.001 (Figure 2E)). Consistent results were obtained by Western blot analysis (Figure 6s). This finding is consistent with the ability of swim stress to potentiate COMT-dependent thermal heat hyperalgesia in female, but not male mice. In the absence of OR486 delivery, swim stress did not alter spinal BDNF expression (Figure 2s).
Figure 2. Stress exacerbates COMT-dependent increases in spinal BDNF expression in females, but not males.
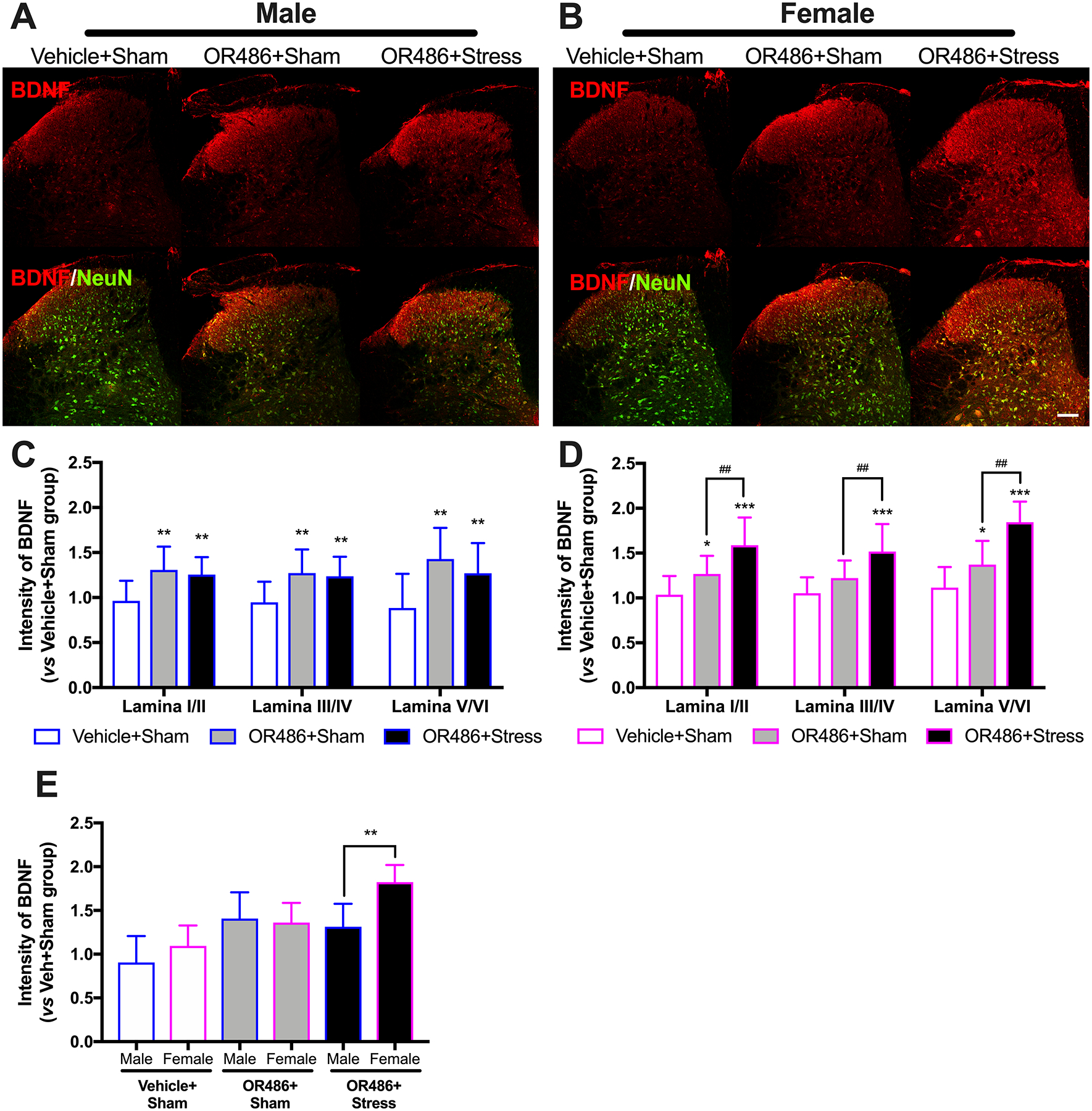
Immunohistochemical staining shows expression of spinal BDNF on day 14 in (A) males and (B) females receiving the COMT inhibitor OR486 or vehicle in the absence or presence of swim stress. Quantitative analysis of immunofluorescence intensity demonstrates that sustained OR486 delivery results in increased expression of BDNF in spinal dorsal horn in both (C) males and (D) females, and that swim stress potentiates COMT-dependent increases in spinal BDNF expression in females, but not males. (E) The potentiation of COMT-dependent increases in spinal BDNF expression by stress in females vs males is confirmed by analysis of between-subjects factors in the same ANOVA. Data are shown as Mean ± SD, N=6–8 per group, *P < 0.1, **P < 0.01, ***P < 0.001 different from the Vehicle+Sham group; ##P < 0.01, different from the OR486+Sham group. Scale bar = 100 μm.
3.3. Effect of Stress on COMT-dependent Increases in Spinal GR Expression in Males and Females
Glucocorticoid-GR interactions promote neuronal plasticity and maintain pain following injury and/or stress.[1; 54; 55] Here, we evaluated spinal GR expression in our mouse model of functional pain in the presence and absence of stress. We found that sustained systemic delivery of OR486 led to increases in spinal GR in both males and females. Compared to the Vehicle+Sham group, male and female mice in the OR486+Sham group exhibited increased GR expression in lamina I-VI of the spinal dorsal horn (Two-way ANOVA for group × lamina. Males: F1,90 = 95.33, P < 0.001 (Fig 3A,C) and females: F1,90 = 96.07, P < 0.001 (Fig 3B,D)). In contrast to BDNF, GR expression was not potentiated by stress in females or males, and no differences were observed between males and females (Figure 3E). In the absence of OR486 delivery, swim stress did not alter spinal GR expression (Figure 3s). Again, consistent results were obtained by Western blot analysis (Figure 6s).
Figure 3. COMT inhibition leads to increases in spinal GR expression in a stress- and sex-independent manner.
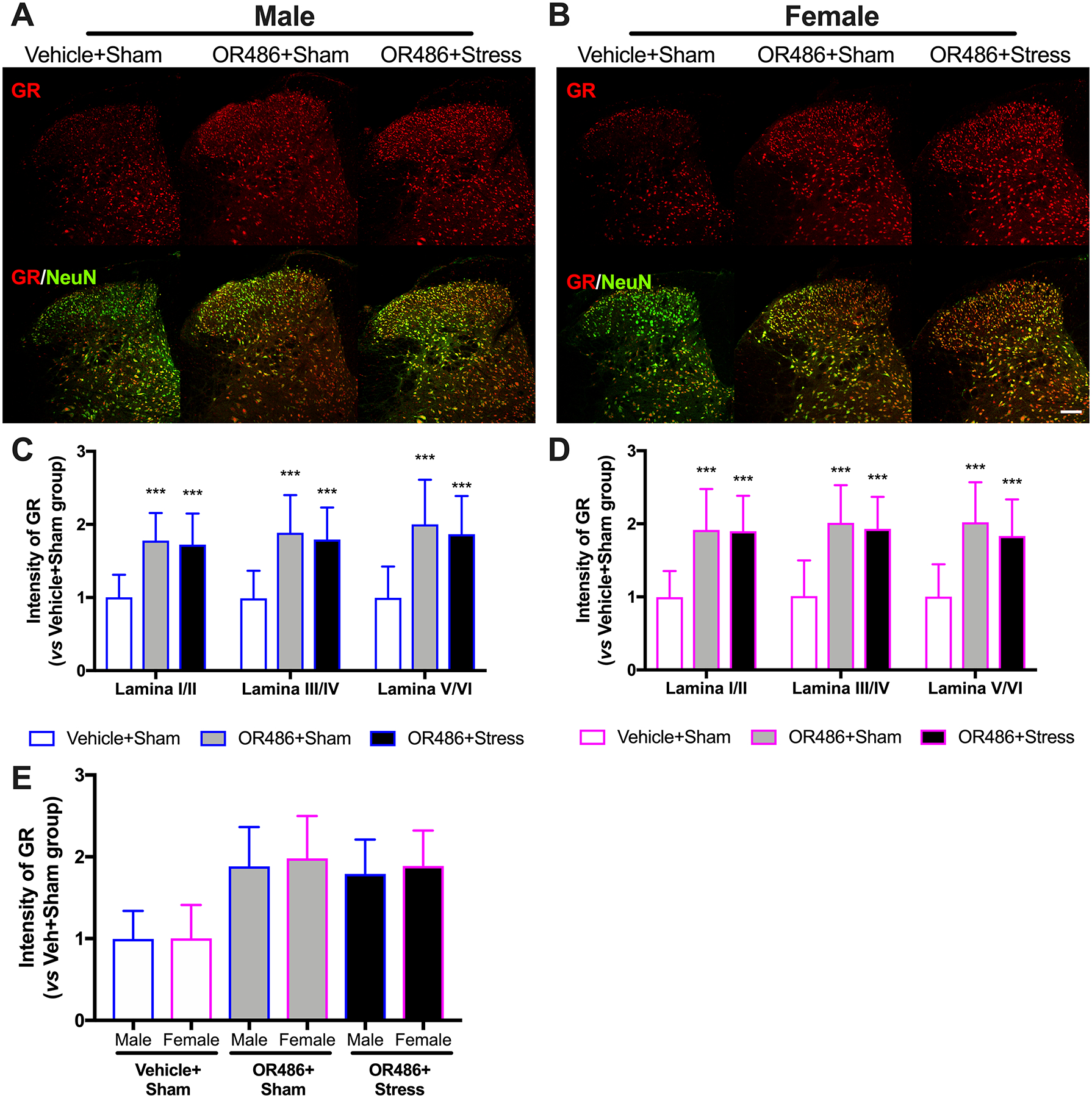
Immunohistochemical staining shows expression of spinal GR on day 14 in (A) males and (B) females receiving the COMT inhibitor OR486 or vehicle in the absence or presence of swim stress. Quantitative analysis of immunofluorescence intensity demonstrates that sustained OR486 delivery leads to increased expression of GR in spinal dorsal horn in both (C) males and (D) females, and that swim stress does not alter COMT-dependent increases in spinal GR expression. (E) No sexually dimorphic effects were observed following analysis of between-subjects factors in the same ANOVA. Data are shown as Mean ± SD, N=6–8 per group, ***P < 0.001 different from the Vehicle+Sham group. Scale bar = 100 μm.
3.4. Effect of COMT Inhibition on Stress-induced Depressive-like Behavior in Males and Females
We evaluated the independent and joint contributions of low COMT and stress on depressive-like behavior. We found that that the 3-day repeated swim stress paradigm, but not sustained systemic delivery of OR486, produced a significant increase in depressive-like behavior during the TST, but only among females (One-way ANOVA for group. F3,35 = 9.8, P < 0.001 (Figure 4A)). Compared to females in the Vehicle+Sham group, those in the Vehicle+Stress group exhibited significant increases in immobility time. Next, we found that repeated swim stress together with sustained OR486 delivery produced significantly greater depressive-like behavior than swim stress alone. Females in the OR486+Stress group exhibited significant increases in immobility time during the TST compared to those in the Vehicle+Stress group. Males in the OR486+Stress group did not differ from those in the Vehicle+Stress group, although they did exhibit increased immobility time during the TST compared to those in the Vehicle+Sham group. The greater impact of repeated swim stress and sustained OR486 delivery on depressive-like behavior in females was also observed during the final 10 minutes of the third and final day of the swim stress paradigm (One-way ANOVA for group. F3,45 = 5.7, P < 0.003 (Figure 4B)). During the swim task, immobility time for females in the OR486+Stress group was 40% greater than that for males. Thus, OR486 delivery exacerbates stress-induced depressive-like behavior, especially in females.
Figure 4. COMT inhibition exacerbates stress-induced depressive-like behavior, especially in females.
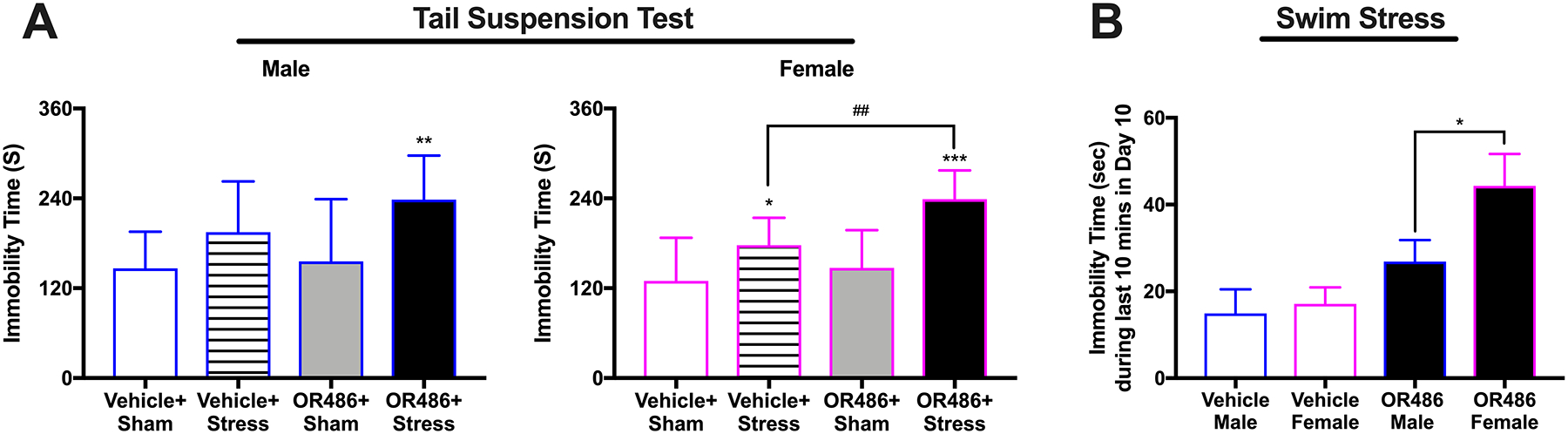
(A) Swim stress leads to increased immobility time during the tail suspension test, which is potentiated by sustained OR486 delivery, in males and females. Data are shown as Mean ± SD, N=8–13 per group, *P < 0.05, **P < 0.01, ***P < 0.001 different from the Vehicle+Sham group; ##P < 0.01, different from the Vehicle+Stress group.
(B) Compared to males, female mice in the OR486+Stress group exhibit a 4-fold greater increase in immobility time during the swim task on day 3. Data are shown as Mean ± SD, N=8–13 per group, *P < 0.05.
3.5. Effect of COMT Inhibition on Stress-dependent Decreases in Hippocampal BDNF Expression in Males and Females
BDNF not only acts as a pronociceptive factor, but also serves in depression. Here we assessed the expression of BDNF in the hippocampus CA1 area from the four different groups. We found that swim stress led to decreases in hippocampal BDNF in both male and females (One-way ANOVA for group. Males: F2,30 = 11.16, P < 0.001 (Fig 5B,D) and females: F2,27 = 32.71, P < 0.001 (Fig 5C,E)), and OR486 delivery exacerbated this decrease in females, but not males. Compared to the Vehicle+Sham group, mice in Vehicle+Stress group exhibited decreased BDNF expression in the hippocampus. Compared to mice in the Vehicle+Stress group, female, but not male mice, in the OR486+Stress group exhibited even lower decreases in hippocampal BDNF expression. This finding is consistent with the ability of COMT inhibition to exacerbate stress-dependent depressive-like behaviors mostly in female, but not male mice. Analysis of between-subjects factors in the same ANOVA revealed that the potentiation of OR486-dependent increases in hippocampal BDNF expression by stress in female vs male mice is significant (One-way ANOVA for group. F5,57 = 18.67, P < 0.001 (Figure 5F)). In the absence of swim stress, OR486 delivery also resulted in a modest decrease in hippocampal BDNF expression (One-way ANOVA for group. F1,38 = 20.64 (Figure 4s)), with no sexual dimorphism observed (Figure 4s). Consistent results were obtained by Western blot analysis (Figure 6s).
Figure 5. COMT inhibition exacerbates stress-dependent decreases in expression of hippocampal BDNF in females, but not males.
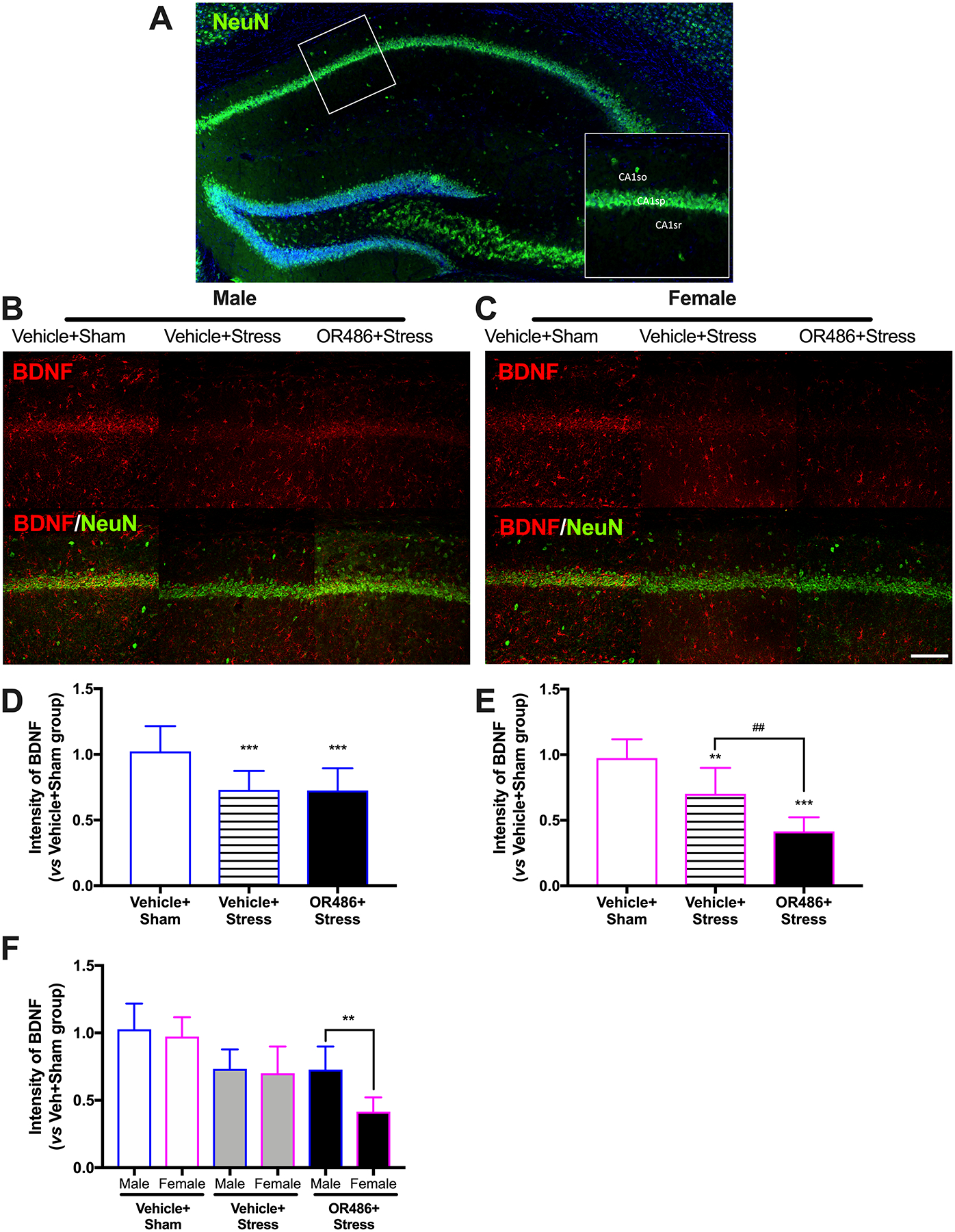
(A) A gross hippocampal image stained with NeuN (Green) depicts the CA1 area where we focused our analyses. The pyramidal layer (CA1sp) is shown as the transverse dot line in the middle, the area below CA1sp is stratum radiatum (CA1sr), and the area above CA1sp is stratum oriens (CA1so). Immunohistochemical staining shows expression of BDNF in the hippocampal CA1 area on day 14 in (B) males and (C) females receiving the COMT inhibitor OR486 or vehicle in the absence or presence of swim stress. Quantitative analysis of immunofluorescence intensity demonstrates that swim stress leads to decreased expression of hippocampal BDNF in both (D) male and (E) female mice, and that sustained OR486 delivery potentiates stress-induced decreases in hippocampal BDNF expression in females, but not males. (F) The potentiation of stress-dependent increases in spinal BDNF expression by OR486 in females vs males is confirmed by analysis of between-subjects factors in the same ANOVA. Data are shown as Mean ± SD, N=6–8 per group, **P < 0.01, ***P < 0.001 different from the Vehicle+Sham group; ##P < 0.01 different from the Vehicle+Stress group. Scale bar = 100 μm.
3.6. Effect of COMT on Stress-dependent Increases in Hippocampal GR Expression in Males and Females
Finally, we examined hippocampal GR expression in a stress-induced depression model in the presence and absence of COMT inhibition. We found that swim stress resulted in increased hippocampal GR expression in both males and females (One-way ANOVA for group. males: F2,18 = 7.246, P < 0.01 (Fig 6A,C) and females: F2,18 = 14.95, P < 0.01 (Fig 6B,D)). Compared to the Vehicle+Sham group, mice in the Vehicle+Stress group exhibited higher hippocampal GR expression. In contrast to BDNF, hippocampal GR expression was not potentiated by COMT inhibition either in females or in males, and no differences were observed between males and females (Figure 6E). In the absence of swim stress, COMT inhibition did not alter hippocampal GR expression (Figure 5s). Again, consistent results were obtained by Western blot analysis (Figure 6s).
Figure 6. Stress leads to increases in hippocampal GR expression in a COMT- and sex-independent manner.
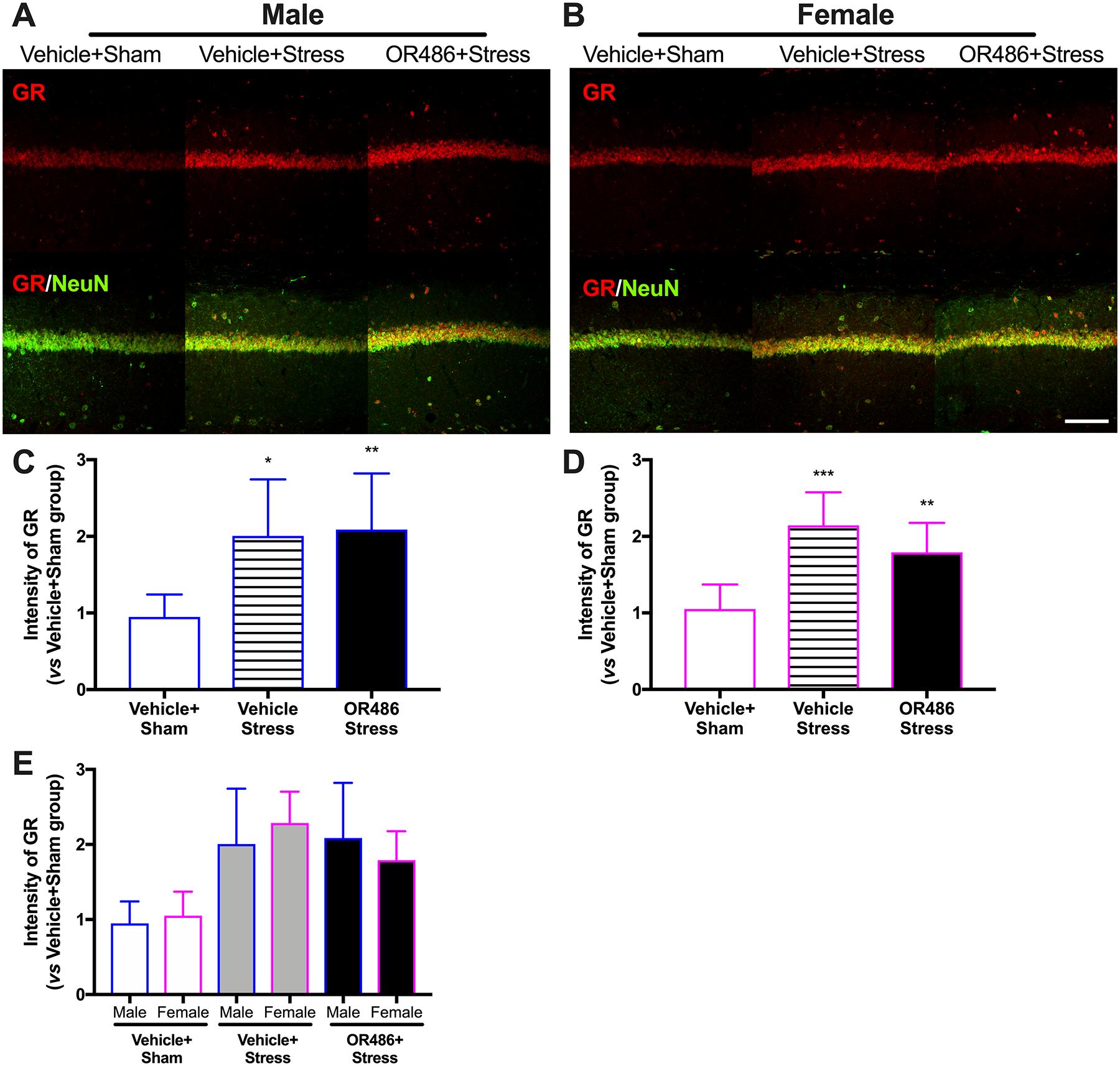
Immunohistochemical staining shows expression of GR in the hippocampal CA1 area on day 14 in (A) males and (B) females receiving the COMT inhibitor OR486 or vehicle in the absence or presence of swim stress. Quantitative analysis of immunofluorescence intensity demonstrates that swim stress leads to increased expression of hippocampal GR in both (C) male and (D) female mice, and that sustained OR486 delivery does not alter stress-dependent increases in hippocampal GR expression. (E) No sexually dimorphic effects were observed following analysis of between-subjects factors in the same ANOVA. Data are shown as Mean ± SD, N=7 per group, *P < 0.1, **P < 0.01, ***P < 0.001 different from the Vehicle+Sham group. Scale bar = 100 μm.
4. Discussion
Accumulating evidence implicates COMT and stress in the etiology of FPS and comorbid depression, which are conditions that predominantly affect females. However, the independent and joint contributions of COMT and stress to these conditions have not been examined. Here, we demonstrated that repeated swim stress potentiates the effect of low COMT on functional pain and that low COMT potentiates the effect of stress on depressive-like behavior. The joint effects of low COMT and stress on functional pain and depressive-like behavior were significantly greater in females relative to males. Consistent with behavioral data, we found that stress potentiates COMT-dependent increases in spinal BDNF and that low COMT potentiates stress-dependent decreases in hippocampal BDNF in females, but not males. Thus, females are more susceptible to the additive effects of factors that increase catecholaminergic tone and alter BDNF signaling.
4.1. Swim Stress Exacerbates COMT-dependent Functional Pain
Many patients with FPS express low COMT activity genetic variants,[53; 66; 81] which are associated with increased circulating catecholamine levels and increased pain evoked by mechanical and thermal stimuli.[24; 113] In previous pre-clinical studies we demonstrated that increased basal catecholamine levels resulting from low COMT drive pain through activation of peripheral βARs and downstream inflammatory mediators that increase nociceptor and glial cell activity.[38; 46; 111] As pain responses in patients with FPS are enhanced following stressful events which potentiate catecholamine release from sympathetic nerves, here we sought to investigate the influence of stress in our mouse model of FPS.[64; 78] First, we evaluated the effects of stress in vehicle-treated mice, and found that swim stress produced mechanical and thermal heat pain in males and females comparable to that produced by OR486. This finding is in line with results from clinical studies demonstrating a causative role for stress in FPS such as fibromyalgia, [104] and pre-clinical studies demonstrating that repeated stress results in long-lasting pain via activation of sensory neurons at spinal and supraspinal sites.[39; 71; 72]
Next, we evaluated the effects of stress in OR486-treated mice, and found that swim stress significantly increased COMT-dependent mechanical and thermal heat pain in males and females. In line with our previous studies, the degree of pain in the OR486+Sham group did not differ between males and females.[17] However, the degree of thermal heat pain in the OR486+Stress group was significantly greater in females. While stress has been shown to enhance pain responses in models of neuropathic pain,[11; 51] this is the first pre-clinical study to demonstrate the significant and sexually dimorphic impact of stress on functional pain.
4.2. COMT Inhibition Exacerbates Stress-induced Depressive-like Behavior
Among patients with chronic FPS, the prevalence of depression ranges from 30–80%.[15; 32; 93; 107] As depression is a major comorbidity of FPS, we sought to investigate the influence of COMT inhibition and stress on depressive-like behavior. First, we evaluated the effects of stress in vehicle-treated mice. In line with results from clinical studies demonstrating that stress is a major contributing factor to the occurrence of depression, [19; 63; 75] we found that swim stress led to depressive-like behavior, but only in females.
Next, we evaluated the effects of stress in OR486-treated mice. We found that COMT inhibition significantly increased stress-dependent depressive-like behavior in females, but not males. This finding is consistent with a growing literature that individuals with low COMT activity variants are more susceptible to the negative consequences of stress on depression. Individuals with the low COMT activity variant are at greater risk for depression during childhood[22] and adulthood,[21; 45; 58] and their depressive symptoms are associated with precipitating stressors. Meanwhile, transgenic mice overexpressing the human high COMT activity variant exhibit diminished stress responses.[68] Our results together with published findings suggest that depression and FPS share a common etiology linked to abnormalities in catecholaminergic tone.[30; 48; 88; 108]
4.3. COMT Inhibition and Stress, Together, Unmask Sexual Dimorphism in Functional Pain and Depressive Behavior
FPS and depression predominantly affect females.[3; 7; 8; 33; 56; 65] Females are, on average, 3 times more likely to be diagnosed with FPS[29; 40; 95] and 2 times more likely to experience depression.[12; 83; 100; 101] Here, we found that OR486 and swim stress together lead to enhanced thermal heat hyperalgesia and depressive-like behavior only in females. The unmasking of sexual dimorphism in pain and depressive phenotypes required both COMT inhibition and stress. This interesting discovery could be due to inherent sex differences in catecholamine tone. COMT activity levels are lower in peripheral and brain tissues of females,[10; 16] and COMT-related sex differences in nociception, cognition, anxiety and other behavioral traits have also been observed.[7; 31; 37; 68; 103] The co-occurance of catecholamine-dependent pain and depressive-like behavior, which is more pronounced in females, suggests common neurobiologic alterations in regions that mediate these behaviors.
4.4. BDNF is a Sex-specific Biomarker Correlated with Functional Pain and Depressive-like Behavior
Following assessment of behavior on day 14, spinal and hippocampal tissues were collected to determine the independent and joint effects of COMT inhibition and stress on BDNF and GR expression. BDNF is a neurotrophin essential for many aspects of central nerve system (CNS) function, including activity-dependent forms of synaptic plasticity.[43] A large body of literature has also demonstrated a major role for BDNF in pain[57; 79] and depressive disorders.[44; 69; 109] Here, we first examined the independent effects of COMT inhibition and stress on BDNF expression in pain-relevant spinal regions. Compared to mice in the Vehicle+Sham group, males and females in the OR486+Sham, but not the Vehicle+Stress, group exhibited significant increases in spinal BDNF expression. This suggests that spinal BDNF is a marker of pain versus stress, and is consistent with findings from published reports demonstrating that increases in spinal BDNF expression contribute to pain hypersensitivity in pre-clinical models of surgical incision,[50] orthopedic surgery,[110] and neuropathic pain.[112]
Next, we examined the independent effects of COMT inhibition and stress on BDNF expression in the depression-relevant CA1 hippocampal region. Compared to mice in the Vehicle+Sham group, males and females in the Vehicle+Stress, but not OR486+Sham, group exhibited significant increases in hippocampal BDNF expression. This finding suggests that hippocampal BDNF is a marker of stress/depression versus pain, and is in line with findings from clinical and pre-clinical studies demonstrating that depressed individuals exhibit decreased BDNF expression in the hippocampus [26; 74; 85]. The utility of hippocampal BDNF as a marker of stress/depression is underscored by its correlation with disease severity and antidepressant treatment response.[4; 90; 109]
Finally, we examined the joint effects of COMT inhibition and stress on BDNF expression in spinal and hippocampal tissues. We found that mice in the OR486+Stress group exhibited marked increases in spinal BDNF expression compared to the OR486+Sham group and marked decreases in hippocampal BDNF expression compared to the Vehicle+Stress group. Consistent with our behavioral data, this effect was only observed in females. Region- and sex-specific alterations in BDNF expression have been shown to occur in different neurological disorders. For example, chronic prenatal stress leads to increased spinal BDNF expression in correlation with increased visceral hypersensitivity in female, but not male rats.[105] Similarly, maternal separation leads to decreased hippocampal BDNF expression alongside anhedonia in mature female but not male rats.[42] Here, we provide the first demonstration that BDNF expression is altered in a region- and sex-specific manner following COMT inhibition and stress. Thus, BDNF might act as a predicative marker for sexual dimorphism.
4.5. GR Expression is Correlated with Functional Pain and Depressive-like Behavior
Glucocorticoid activation of GR plays a critical role in neuroplasticity related to both neuropathic pain [97–99] depression.[2] Here, we first examined the independent effects of COMT inhibition and stress on GR expression in the spinal cord. Compared to mice in the Vehicle+Sham group, males and females in the OR486+Sham, but not Vehicle+Stress, group exhibited significant increases in spinal GR expression. This finding suggests that spinal GR is a marker of pain versus stress, and is consistent with findings from studies demonstrating increased GR expression in the spinal dorsal horn of rodents following nerve injury.[86; 98; 99]
Next, we examined the independent effects of COMT inhibition and stress on GR expression in the CA1 hippocampal region. Compared to mice in the Vehicle+Sham group, males and females in the Vehicle+Stress, but not OR486+Sham, group exhibited significant increases in hippocampal GR expression. This finding suggests that hippocampal GR is a marker of stress/depression versus pain, and is consistent with those from the previous studies showing increased hippocampal GR expression in different types of depression.[35; 91; 96] Interestingly, GR can down-regulate BDNF expression in the hippocampus. Thus, the crosstalk between GR and BDNF signaling pathways might play a major role in physiology and pathology of depression.[2]
Finally, we assessed the joint effects of COMT inhibition and stress on GR expression in spinal and hippocampal tissues. We found that males and females in the OR486+Stress group exhibited comparable increases in spinal GR expression compared to the OR486+Sham group and in hippocampal GR expression compared to the Vehicle+Stress group. Thus, stress does not influence COMT-dependent increases in spinal GR and COMT inhibition does not influence stress-dependent increases in hippocampal GR expression.
5. Conclusions
Our findings suggest that functional pain syndromes and depressive disorders, which predominantly affect females, share a common etiology linked to heightened catecholaminergic tone. Thus, therapies that normalize catecholamine signaling may prove useful in the treatment of comorbid functional pain and depression. Further, we identified BDNF as a sex-specific biomarker correlated with functional pain and depressive behavior.
Supplementary Material
Acknowledgements
This work was funded by the NIH/NINDS R01 NS109541 and NIH/NINDS R03 NS106166 to A.N.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- [1].Alexander JK, DeVries AC, Kigerl KA, Dahlman JM, Popovich PG. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain, behavior, and immunity 2009;23(6):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011;36(3):415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Asher M, Asnaani A, Aderka IM. Gender differences in social anxiety disorder: A review. Clin Psychol Rev 2017;56:1–12. [DOI] [PubMed] [Google Scholar]
- [4].Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain research 2008;1211:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barbosa FR, Matsuda JB, Mazucato M, de Castro Franca S, Zingaretti SM, da Silva LM, Martinez-Rossi NM, Junior MF, Marins M, Fachin AL. Influence of catechol-O-methyltransferase (COMT) gene polymorphisms in pain sensibility of Brazilian fibromialgia patients. Rheumatol Int 2012;32(2):427–430. [DOI] [PubMed] [Google Scholar]
- [6].Baumann C, Klauke B, Weber H, Domschke K, Zwanzger P, Pauli P, Deckert J, Reif A. The interaction of early life experiences with COMT val158met affects anxiety sensitivity. Genes Brain Behav 2013;12(8):821–829. [DOI] [PubMed] [Google Scholar]
- [7].Belfer I, Segall SK, Lariviere WR, Smith SB, Dai F, Slade GD, Rashid NU, Mogil JS, Campbell CM, Edwards RR, Liu Q, Bair E, Maixner W, Diatchenko L. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain 2013;154(8):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berkley KJ. Sex differences in pain. Behav Brain Sci 1997;20(3):371–380; discussion 435–513. [DOI] [PubMed] [Google Scholar]
- [9].Bote ME, Garcia JJ, Hinchado MD, Ortega E. An exploratory study of the effect of regular aquatic exercise on the function of neutrophils from women with fibromyalgia: role of IL-8 and noradrenaline. Brain Behav Immun 2014;39:107–112. [DOI] [PubMed] [Google Scholar]
- [10].Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clinical pharmacology and therapeutics 1990;48(4):381–389. [DOI] [PubMed] [Google Scholar]
- [11].Bravo L, Alba-Delgado C, Torres-Sanchez S, Mico JA, Neto FL, Berrocoso E. Social stress exacerbates the aversion to painful experiences in rats exposed to chronic pain: the role of the locus coeruleus. Pain 2013;154(10):2014–2023. [DOI] [PubMed] [Google Scholar]
- [12].Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lepine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC medicine 2011;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp 2012(59):e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp 2012(59):e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Canales GT, Guarda-Nardini L, Rizzatti-Barbosa CM, Conti PCR, Manfredini D. Distribution of depression, somatization and pain-related impairment in patients with chronic temporomandibular disorders. Journal of applied oral science : revista FOB 2019;27:e20180210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen LX, Fang Q, Chen Q, Guo J, Wang ZZ, Chen Y, Wang R. Study in vitro and in vivo of nociceptin/orphanin FQ(1–13)NH2 analogues substituting N-Me-Gly for Gly2 or Gly3. Peptides 2004;25(8):1349–1354. [DOI] [PubMed] [Google Scholar]
- [17].Ciszek BP, O’Buckley SC, Nackley AG. Persistent Catechol-O-methyltransferase-dependent Pain Is Initiated by Peripheral beta-Adrenergic Receptors. Anesthesiology 2016;124(5):1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clauw DJ. Fibromyalgia: a clinical review. JAMA : the journal of the American Medical Association 2014;311(15):1547–1555. [DOI] [PubMed] [Google Scholar]
- [19].Cohen H, Kaplan Z, Matar MA, Loewenthal U, Zohar J, Richter-Levin G. Long-lasting behavioral effects of juvenile trauma in an animal model of PTSD associated with a failure of the autonomic nervous system to recover. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 2007;17(6–7):464–477. [DOI] [PubMed] [Google Scholar]
- [20].Cohen H, Neumann L, Glazer Y, Ebstein RP, Buskila D. The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val(158) met and fibromyalgia. Clinical and experimental rheumatology 2009;27(5 Suppl 56):S51–56. [PubMed] [Google Scholar]
- [21].Comasco E, Sylven SM, Papadopoulos FC, Sundstrom-Poromaa I, Oreland L, Skalkidou A. Postpartum depression symptoms: a case-control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatric genetics 2011;21(1):19–28. [DOI] [PubMed] [Google Scholar]
- [22].Conway CC, Hammen C, Brennan PA, Lind PA, Najman JM. Interaction of chronic stress with serotonin transporter and catechol-O-methyltransferase polymorphisms in predicting youth depression. Depress Anxiety 2010;27(8):737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005;438(7070):1017–1021. [DOI] [PubMed] [Google Scholar]
- [24].Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 2005;14(1):135–143. [DOI] [PubMed] [Google Scholar]
- [25].Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine committee. JAMA 2014;312(15):1507–1508. [DOI] [PubMed] [Google Scholar]
- [26].Elfving B, Plougmann PH, Muller HK, Mathe AA, Rosenberg R, Wegener G. Inverse correlation of brain and blood BDNF levels in a genetic rat model of depression. Int J Neuropsychopharmacol 2010;13(5):563–572. [DOI] [PubMed] [Google Scholar]
- [27].Evaskus DS, Laskin DM. A biochemical measure of stress in patients with myofascial pain-dysfunction syndrome. J Dent Res 1972;51(5):1464–1466. [DOI] [PubMed] [Google Scholar]
- [28].Fernandez-de-Las-Penas C, Ambite-Quesada S, Gil-Crujera A, Cigaran-Mendez M, Penacoba-Puente C. Catechol-O-methyltransferase Val158Met polymorphism influences anxiety, depression, and disability, but not pressure pain sensitivity, in women with fibromyalgia syndrome. J Pain 2012;13(11):1068–1074. [DOI] [PubMed] [Google Scholar]
- [29].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. The journal of pain : official journal of the American Pain Society 2009;10(5):447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gaszner P, Miklya I. Major depression and the synthetic enhancer substances, (−)-deprenyl and R-(−)-1-(benzofuran-2-yl)-2-propylaminopentane. Progress in neuro-psychopharmacology & biological psychiatry 2006;30(1):5–14. [DOI] [PubMed] [Google Scholar]
- [31].Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A 1998;95(17):9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression. Pain research and treatment 2012;2012:486590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Consensus Working Group of the Sex G, Pain SIGotI. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132 Suppl 1:S26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int 2003;23(3):104–107. [DOI] [PubMed] [Google Scholar]
- [35].Han QQ, Yang L, Huang HJ, Wang YL, Yu R, Wang J, Pilot A, Wu GC, Liu Q, Yu J. Differential GR Expression and Translocation in the Hippocampus Mediates Susceptibility vs. Resilience to Chronic Social Defeat Stress. Frontiers in neuroscience 2017;11:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;32(1):77–88. [DOI] [PubMed] [Google Scholar]
- [37].Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2008;33(13):3037–3045. [DOI] [PubMed] [Google Scholar]
- [38].Hartung JE, Ciszek BP, Nackley AG. beta2- and beta3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain 2014;155(7):1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hata T, Itoh E, Kawabata A. Changes in CNS levels of serotonin and its metabolite in SART-stressed (repeatedly cold-stressed) rats. Japanese journal of pharmacology 1991;56(1):101–104. [DOI] [PubMed] [Google Scholar]
- [40].Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017;37(9):1527–1539. [DOI] [PubMed] [Google Scholar]
- [41].Hettema JM, An SS, Bukszar J, van den Oord EJ, Neale MC, Kendler KS, Chen X. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol Psychiatry 2008;64(4):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus 2014;24(10):1197–1211. [DOI] [PubMed] [Google Scholar]
- [43].Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience 2001;24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jeanneteau F, Borie A, Chao MV, Garabedian MJ. Bridging the Gap between Brain-Derived Neurotrophic Factor and Glucocorticoid Effects on Brain Networks. Neuroendocrinology 2018. [DOI] [PubMed] [Google Scholar]
- [45].Jimenez KM, Pereira-Morales AJ, Forero DA. Val158Met polymorphism in the COMT gene is associated with hypersomnia and mental health-related quality of life in a Colombian sample. Neuroscience letters 2017;644:43–47. [DOI] [PubMed] [Google Scholar]
- [46].Kim S, Zhang X, O’Buckley SC, Cooter M, Park JJ, Nackley AG. Acupuncture Resolves Persistent Pain and Neuroinflammation in a Mouse Model of Chronic Overlapping Pain Conditions. The journal of pain : official journal of the American Pain Society 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kline RHt, Exposto FG, O’Buckley SC, Westlund KN, Nackley AG. Catechol-O-methyltransferase inhibition alters pain and anxiety-related volitional behaviors through activation of beta-adrenergic receptors in the rat. Neuroscience 2015;290:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kocabas NA. Catechol-O-methyltransferase (COMT) pharmacogenetics in the treatment response phenotypes of major depressive disorder (MDD). CNS & neurological disorders drug targets 2012;11(3):264–272. [DOI] [PubMed] [Google Scholar]
- [49].Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biological psychiatry 2010;67(4):304–308. [DOI] [PubMed] [Google Scholar]
- [50].Li CQ, Xu JM, Liu D, Zhang JY, Dai RP. Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Molecular pain 2008;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li MJ, Liu LY, Chen L, Cai J, Wan Y, Xing GG. Chronic stress exacerbates neuropathic pain via the integration of stress-affect-related information with nociceptive information in the central nucleus of the amygdala. Pain 2017;158(4):717–739. [DOI] [PubMed] [Google Scholar]
- [52].Liu J, Gong P, Gao X, Zhou X. The association between well-being and the COMT gene: Dispositional gratitude and forgiveness as mediators. J Affect Disord 2017;214:115–121. [DOI] [PubMed] [Google Scholar]
- [53].Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995;34(13):4202–4210. [DOI] [PubMed] [Google Scholar]
- [54].Madalena KM, Lerch JK. The Effect of Glucocorticoid and Glucocorticoid Receptor Interactions on Brain, Spinal Cord, and Glial Cell Plasticity. Neural plasticity 2017;2017:8640970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Maiaru M, Tochiki KK, Cox MB, Annan LV, Bell CG, Feng X, Hausch F, Geranton SM. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Sci Transl Med 2016;8(325):325ra319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. The journal of pain : official journal of the American Pain Society 2016;17(9 Suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Malcangio M Spinal mechanisms of neuropathic pain: Is there a P2X4-BDNF controversy? Neurobiol Pain 2017;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mandelli L, Serretti A, Marino E, Pirovano A, Calati R, Colombo C. Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. Int J Neuropsychopharmacol 2007;10(4):437–447. [DOI] [PubMed] [Google Scholar]
- [59].Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 1999;51(4):593–628. [PubMed] [Google Scholar]
- [60].Marbach JJ, Levitt M. Erythrocyte catechol-O-methyltransferase activity in facial pain patients. J Dent Res 1976;55(4):711. [DOI] [PubMed] [Google Scholar]
- [61].Martinez-Lavin M, Vidal M, Barbosa RE, Pineda C, Casanova JM, Nava A. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study [ISRCTN70707830]. BMC Musculoskelet Disord 2002;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Matsuda JB, Barbosa FR, Morel LJ, Franca Sde C, Zingaretti SM, da Silva LM, Pereira AM, Marins M, Fachin AL. Serotonin receptor (5-HT 2A) and catechol-O-methyltransferase (COMT) gene polymorphisms: triggers of fibromyalgia? Rev Bras Reumatol 2010;50(2):141–149. [PubMed] [Google Scholar]
- [63].McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013;79(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. The journal of pain : official journal of the American Pain Society 2011;12(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature reviews Neuroscience 2012;13(12):859–866. [DOI] [PubMed] [Google Scholar]
- [66].Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 2006;314(5807):1930–1933. [DOI] [PubMed] [Google Scholar]
- [67].Nissinen E, Linden IB, Schultz E, Kaakkola S, Mannisto PT, Pohto P. Inhibition of catechol-O-methyltransferase activity by two novel disubstituted catechols in the rat. European journal of pharmacology 1988;153(2–3):263–269. [DOI] [PubMed] [Google Scholar]
- [68].Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2008;28(35):8709–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Park C, Rosenblat JD, Brietzke E, Pan Z, Lee Y, Cao B, Zuckerman H, Kalantarova A, McIntyre RS. Stress, epigenetics and depression: A systematic review. Neuroscience and biobehavioral reviews 2019;102:139–152. [DOI] [PubMed] [Google Scholar]
- [70].Perry F, Heller PH, Kamiya J, Levine JD. Altered autonomic function in patients with arthritis or with chronic myofascial pain. Pain 1989;39(1):77–84. [DOI] [PubMed] [Google Scholar]
- [71].Quintero L, Cuesta MC, Silva JA, Arcaya JL, Pinerua-Suhaibar L, Maixner W, Suarez-Roca H. Repeated swim stress increases pain-induced expression of c-Fos in the rat lumbar cord. Brain research 2003;965(1–2):259–268. [DOI] [PubMed] [Google Scholar]
- [72].Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacology, biochemistry, and behavior 2000;67(3):449–458. [DOI] [PubMed] [Google Scholar]
- [73].Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis 2009;41(11):772–780. [DOI] [PubMed] [Google Scholar]
- [74].Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behavioural brain research 2001;120(1):87–95. [DOI] [PubMed] [Google Scholar]
- [75].Seo JS, Wei J, Qin L, Kim Y, Yan Z, Greengard P. Cellular and molecular basis for stress-induced depression. Molecular psychiatry 2017;22(10):1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sheikh HI, Kryski KR, Kotelnikova Y, Hayden EP, Singh SM. Catechol-O-Methyltransferase gene (val158met) polymorphisms and anxious symptoms in early childhood: The roles of hypothalamus-pituitary-adrenal axis reactivity and life stress. Neurosci Lett 2017;659:86–91. [DOI] [PubMed] [Google Scholar]
- [77].Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain 2013;14(12 Suppl):T20–32 e21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Slade GD, Sanders AE, Ohrbach R, Bair E, Maixner W, Greenspan JD, Fillingim RB, Smith S, Diatchenko L. COMT Diplotype Amplifies Effect of Stress on Risk of Temporomandibular Pain. J Dent Res 2015;94(9):1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Smith PA. BDNF: no gain without pain? Neuroscience 2014;283:107–123. [DOI] [PubMed] [Google Scholar]
- [80].Smith SB, Reenila I, Mannisto PT, Slade GD, Maixner W, Diatchenko L, Nackley AG. Epistasis Between Polymorphisms in COMT, ESR1, and GCH1 Influences COMT Enzyme Activity and Pain. Pain 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Smith SB, Reenila I, Mannisto PT, Slade GD, Maixner W, Diatchenko L, Nackley AG. Epistasis between polymorphisms in COMT, ESR1, and GCH1 influences COMT enzyme activity and pain. Pain 2014;155(11):2390–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Smith SB, Reenila I, Mannisto PT, Slade GD, Maixner W, Diatchenko L, Nackley AG. Epistasis Between Polymorphisms in COMT, ESR1, and GCH1 Influences COMT Enzyme Activity and Pain. Pain 2014;155(11):2390–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci 2008;33(4):331–343. [PMC free article] [PubMed] [Google Scholar]
- [84].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature neuroscience 2015;18(8):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Su CL, Su CW, Hsiao YH, Gean PW. Epigenetic regulation of BDNF in the learned helplessness-induced animal model of depression. J Psychiatr Res 2016;76:101–110. [DOI] [PubMed] [Google Scholar]
- [86].Takasaki I, Kurihara T, Saegusa H, Zong S, Tanabe T. Effects of glucocorticoid receptor antagonists on allodynia and hyperalgesia in mouse model of neuropathic pain. European journal of pharmacology 2005;524(1–3):80–83. [DOI] [PubMed] [Google Scholar]
- [87].Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia. Arthritis and rheumatism 2000;43(4):872–880. [DOI] [PubMed] [Google Scholar]
- [88].Tremblay P, Blier P. Catecholaminergic strategies for the treatment of major depression. Current drug targets 2006;7(2):149–158. [DOI] [PubMed] [Google Scholar]
- [89].Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. The journal of pain : official journal of the American Pain Society 2008;9(10):883–891. [DOI] [PubMed] [Google Scholar]
- [90].Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience 2006;9(4):519–525. [DOI] [PubMed] [Google Scholar]
- [91].van der Doelen RH, Calabrese F, Guidotti G, Geenen B, Riva MA, Kozicz T, Homberg JR. Early life stress and serotonin transporter gene variation interact to affect the transcription of the glucocorticoid and mineralocorticoid receptors, and the co-chaperone FKBP5, in the adult rat brain. Front Behav Neurosci 2014;8:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadoniga JI, Garcia-Fructuoso F, Ramos-Kuri M, Hernandez F, Springall R, Bojalil R, Vallejo M, Martinez-Lavin M. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis research & therapy 2007;9(5):R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Vereckei E, Susanszky E, Kopp M, Ratko I, Czimbalmos A, Nagy Z, Palkonyai E, Hodinka L, Temesvari PI, Kiss E, Toro K, Poor G. Psychosocial, educational, and somatic factors in chronic nonspecific low back pain. Rheumatol Int 2013;33(3):587–592. [DOI] [PubMed] [Google Scholar]
- [94].Vrijsen JN, van Oostrom I, Arias-Vasquez A, Franke B, Becker ES, Speckens A. Association between genes, stressful childhood events and processing bias in depression vulnerable individuals. Genes, brain, and behavior 2014;13(5):508–516. [DOI] [PubMed] [Google Scholar]
- [95].Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The Prevalence and Characteristics of Fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10(9):e0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang Q, Joels M, Swaab DF, Lucassen PJ. Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology 2012;62(1):527–533. [DOI] [PubMed] [Google Scholar]
- [97].Wang QS, Jiang YH, Wang TD, Xiao T, Wang JK. Effects of betamethasone on neuropathic pain in a rat spare nerve injury model. Clinical and experimental pharmacology & physiology 2013;40(1):22–27. [DOI] [PubMed] [Google Scholar]
- [98].Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 2004;24(39):8595–8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang S, Lim G, Zeng Q, Sung B, Yang L, Mao J. Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 2005;25(2):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. Journal of affective disorders 1993;29(2–3):77–84. [DOI] [PubMed] [Google Scholar]
- [101].Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry 1977;34(1):98–111. [DOI] [PubMed] [Google Scholar]
- [102].Wesselmann U, Bonham A, Foster D. Vulvodynia: Current state of the biological science. Pain 2014;155(9):1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].White TP, Loth E, Rubia K, Krabbendam L, Whelan R, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Paillere ML, Nees F, Paus T, Pausova Z, Rietschel M, Robbins T, Smolka MN, Shergill SS, Schumann G, Consortium I. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology 2014;39(11):2560–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Williams DA, Brown SC, Clauw DJ, Gendreau RM. Self-reported symptoms before and after September 11 in patients with fibromyalgia. JAMA : the journal of the American Medical Association 2003;289(13):1637–1638. [DOI] [PubMed] [Google Scholar]
- [105].Winston JH, Li Q, Sarna SK. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2014;26(5):715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp 2015(97). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Alimentary pharmacology & therapeutics 2019. [DOI] [PubMed] [Google Scholar]
- [108].Zar-Kessler CAM, Belkind-Gerson J, Bender S, Kuo BM. Treatment of Functional Abdominal Pain With Antidepressants: Benefits, Adverse Effects, and the Gastroenterologist’s Role. Journal of pediatric gastroenterology and nutrition 2017;65(1):16–21. [DOI] [PubMed] [Google Scholar]
- [109].Zhang JC, Yao W, Hashimoto K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Current neuropharmacology 2016;14(7):721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhang MD, Barde S, Yang T, Lei B, Eriksson LI, Mathew JP, Andreska T, Akassoglou K, Harkany T, Hokfelt TG, Terrando N. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proceedings of the National Academy of Sciences of the United States of America 2016;113(43):E6686–E6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhang X, Hartung JE, Bortsov AV, Kim S, O’Buckley SC, Kozlowski J, Nackley AG. Sustained stimulation of beta2- and beta3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain, behavior, and immunity 2018;73:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhou LJ, Yang T, Wei X, Liu Y, Xin WJ, Chen Y, Pang RP, Zang Y, Li YY, Liu XG. Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain, behavior, and immunity 2011;25(2):322–334. [DOI] [PubMed] [Google Scholar]
- [113].Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 2003;299(5610):1240–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


