Abstract
Background
Dysfunction of small conductance calcium activated potassium (SK) channels plays a vital role in atrial arrhythmogenesis. Amiodarone and dronedarone are the most effective class III antiarrhythmic drugs. It is unclear whether the antiarrhythmic effect of amiodarone and dronedarone is related to SK channel inhibition.
Material/Methods
Tissue samples were obtained from the right atria of 46 patients with normal sinus rhythm and 39 patients with chronic atrial fibrillation. Isolated atrial myocytes were obtained by enzymatic dissociation. KCNN2 (SK2) channels were transiently expressed in human embryonic kidney (HEK)-293 cells. SK currents were recorded using whole-cell conventional patch clamp techniques.
Results
Amiodarone and dronedarone showed a concentration-dependent inhibitory effect on SK currents (IKAS) in atrial myocytes from normal sinus rhythm patients and chronic atrial fibrillation patients. The suppressed efficacy of dronedarone and amiodarone on IKAS was greater in atrial myocytes from chronic atrial fibrillation patients than that from normal sinus rhythm patients. Furthermore, in patients with chronic atrial fibrillation, the IC50 value was 2.42 μM with dronedarone and 8.03 μM with amiodarone. In HEK-293 cells with transiently transfected SK2 channels, both dronedarone and amiodarone had a dose-dependent inhibitory effect on IKAS. The IC50 value was 1.7 μM with dronedarone and 7.2 μM with amiodarone in cells from patients with chronic atrial fibrillation. Compared to amiodarone, dronedarone is more efficacy to inhibit IKAS and could be a potential intervention for patients with chronic atrial fibrillation.
Conclusions
Dronedarone provides a great degree of IKAS inhibition in atrial myocytes from chronic atrial fibrillation than amiodarone. IKAS might be a potential target of amiodarone and dronedarone for the management of chronic atrial fibrillation.
MeSH Keywords: Amiodarone, Atrial Fibrillation, Patch-Clamp Techniques, Small-Conductance Calcium-Activated Potassium Channels
Background
Atrial fibrillation (AF), the most common arrhythmia in clinical practice, has a high morbidity and mortality rate [1,2]. The prevalence of AF increases with age [3]. AF treatment is considered one of the most complicated challenges in clinic management. Pharmacology antiarrhythmic therapy is not the first-line treatment strategy due to side effects and risk of heart disease; it is usually reserved for patients who have weak effects by catheter ablation or combination therapy [4–6]. Rhythm control by antiarrhythmics contributes to improving symptoms in AF patients. The class III antiarrhythmic drug amiodarone is a multiple channel blocker that is used as a potassium channel blocker in acute and chronic AF treatment. Furthermore, chronic treatment with amiodarone shows more potent prolongation of the effective refractory period (ERP) in the atria compared to the ventricle [7]. However, extra-cardiac side effects limit its widespread use in clinical AF treatment [8–11]. It has been demonstrated that iodide atoms in amiodarone molecular structure induced extracardiac adverse effects: thyroid and pulmonary toxicity [12,13]. Dronedarone is an amiodarone analogue and has been synthesized with a noniodinated benzofuran derivative molecular structure to avoid these adverse effects. The short-term electrophysiological effects of dronedarone have been shown to be similar to those of amiodarone. Several studies found that the effects of dronedarone-inhibited ion channels (acetylcholine-sensitive potassium channel, IK,Ach) is superior to those of amiodarone [14]. Nevertheless, some studies found that acute treatment by dronedarone is inferior to amiodarone in terminating and preventing AF in canine atria [15]. Hence, the difference in antiarrhythmic efficacy of treatment between amiodarone and dronedarone is still insufficiently understood. Small conductance calcium-activated potassium channels (SK channels) are voltage-independent calcium-activated channels that play a vital role in the late repolarization phase of action potential. Furthermore, the role of SK channels is more important in the atria compared to the ventricle. Genetic knock-out and suppression of SK2 channels result in a remarkable prolongation of action potential duration (APD) [16,17]. Our group found that CaMKII activation induced SK currents to be augmented in humans with AF [18,19]. Other studies also demonstrated that SK channel inhibition contributed to relieving atrial arrhythmia by prolonging APD and ERP [17,20–22]. Herein, as one of the atrial favorite channels (the ultra-rapidly activating delayed rectifier potassium current IKur, the acetylcholine-activated potassium current IKACh and SK channels) [1,23,24], theoretically pharmacological inhibition of SK channels could be effective for atrial fibrillation alleviation. In fact, recent studies have demonstrated that targeting SK channels is beneficial for relieving AF in a number of animal AF models [20,25]. Some studies also analyzed whether class III antiarrhythmic agents targeted SK channels. Nonetheless, the results are various [26,27]. Amiodarone and dronedarone are the most common class III antiarrhythmic drugs, and the antiarrhythmic effects in targeting SK channels are inconsistent in published literatures. The effects of amiodarone and dronedarone on SK channels have not been reported in human atrial myocytes. The purpose of this study was to determine the different efficacies of amiodarone and dronedarone on SK channels from normal sinus rhythm (NSR) and CAF patients, and on the SK2 channel expressed in HEK-293 cells.
Material and Methods
Human tissue samples
Tissue samples from patients’ right atrial from normal sinus rhythm (NSR, n=46) and chronic atrial fibrillation (CAF, n=39) were obtained during cardiac surgery for cardiopulmonary bypass. CAF patients had been diagnosed prior to the sampling by electrocardiogram. The experimental protocols were approved by the Ethics Committee of Affiliated Hospital, Southwest Medical University (Approval No. L2019003). Each patient gave written informed consent.
Isolation of human atrial cardiomyocytes
Cardioplegic solution used for cell isolation was composed of following: 10 mM, taurine, 5 mM adenosine, 8 mM MgSO4, 10 mM HEPES, 50 mM KH2PO4, 100 mM mannitol, 140 mM glucose, (pH 7.4 adjusted with KOH).
The Ca2+-free solution contained the following ingredients: 137 mM NaCl, 1 mM MgSO4, 5 mM KH2PO4, 5 mM HEPES, 10 mM taurine, and 10 mM glucose, (pH 7.4 adjusted with NaOH).
Kraft-Brühe (KB) storage solution contained the following ingredients: 20 mM KCl, 10 mM KH2PO4, 25 mM glucose, 5 mM mannitol, 1 mM albumin, 70 mM L-glutamic acid, 10 mM β-hydroxybutyrate acid, 10 mM taurine, 0.5 mM EGTA, (pH 7.4 adjusted with KOH)
Human cardiomyocytes from right atrial tissues were obtained by a modulated 2-step enzymatic digestion method [28]. Atrial samples were obtained and immediately transferred from operating room to the laboratory in a cold, oxygenated cardioplegic solution. Tissue blocks were cut into 1 mm3 pieces in cardioplegic solution and then were incubated (37°C, 100% O2) with a Ca2+-free solution containing proteinase (type XXIV, 5 units/mL, Sigma) and collagenase (type V, 150 units/mL, Sigma) with stirring for 35–45 minutes. Subsequently, the atrial pieces were transferred to the Ca2+-free solution containing collagenase (type V, 150 units/mL, Sigma) for 5–10 minutes, and this step was repeated 2 times. Cardiomyocytes were harvested by centrifugation at 400 rpm for 3 minutes, and then kept in Kraft-Brühe (KB) storage solution for at least 1 hour before obtaining electrophysiology recording. Rod-shaped elongated myocytes with clear cross striations were selected to electrophysiological experiments.
Cell culture and gene transfection
Human embryonic kidney-293 (HEK-293) cells were obtained from ATCC (USA), which were cultured in Dulbecco’ modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C with a humidified 5% CO2 atmosphere. The human KCNN2 (SK2) gene was cloned into the pCDNA3 mammalian expression vector and was tagged with green fluorescent protein (GFP). After the HEK-293 cells reached 80% confluent in 6-well dishes, the constructed plasmid (1 μg) was transfected into HEK-293 cells using lipofectamine 3000 (Invitrogen) for 48 hours. The transfection efficiency of the SK2 (KCNN2) gene was determined by virtue of the GFP expression. HEK-293 cells tagged with the GFP (green color) were chosen to patch clamp experiment.
Conventional whole-cell patch clamp technique
Bath solution used for atrial myocytes whole-cell recording: 140 mM NMG, 4 mM KCl, 1 mM MgCl2, 5 mM glucose, 10 mM HEPES, (pH 7.4 adjusted with HCl).
Pipette solution used for atria myocytes whole-cell recording: 144 mM potassium gluconate, 1.15 mM MgCl2,, 5 mM EGTA, 10 mM HEPES, (pH 7.4 adjusted with KOH).
The extracellular solution for IKAS recording in HEK-293 cells contained: 140 mmol/L D-aspartic acid, 40 mmol/L KOH, 100 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L HEPES (pH 7.4 adjusted with KOH).
The pipette solution for IKAS recording in HEK-293 cells contained (in mmol/L): 100 mmol/L D-aspartic acid, 100 mmol/L KOH, 40 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L EGTA, 10 mmol/L HEPES, (pH 7.2 adjusted with KOH).
Membrane currents [29] were measured by using AXON 700B amplifier (AXON Instruments, USA) and an Analog to Digital-Digital to Analog (AD-DA) converter. Pipette electrodes were made from borosilicate glass electrodes (outer diameter 1 mm) using a micropipette Puller (Model P-97, Sutter Instrument Co., USA). The microelectrode resistance of the tip was approximately 2–4 MΩ in the bath solution. The series resistance was compensated up to 70% to 80%. The sampling frequency was 10 kHz. Data were acquired and stored using Clamplex 10, and analyzed using Clampfit 10.4 (Axon Instrument, USA) and plotted using Origin 8.0 software (OriginLab Corporation, USA). Whole-cell currents recording performed from human atrial myocytes with a depolarizing step pulse from −130 mV to +60 mV with a holding potential of −60 mV. In HEK-293 cells, symmetrical high potassium extracellular and intracellular solutions were used for recording whole-cell currents. IKAS was elicited by using a voltage ramp protocol from −130 mV to +60 mV for 300 ms with a holding potential of 0 mV.
Data analysis
Data are reported as mean±standard error of the mean (SEM). Statistical analysis was performed using Clampfit 10.4 and Origin 8.0 (OriginLab, USA) software. Currents were normalized by cell membrane capacitance to correct for different cell sizes. For comparison between the 2 conditions, we applied an unpaired t-test. For comparisons among multiple groups, one-way ANOVA was used and was followed by Bonferroni post-test or Newman Keuls correction. Categorical data were compared by Fisher’s exact test. P<0.05 was considered significant.
Results
Patient characteristics
The patients’ information is summarized in Table 1. The patients in CAF group were older than those in NSR group (P<0.05). The left atrial diameter (LAD) in CAF group was larger than that in NSR group (P<0.05). The significant difference was also found in cardiac function between groups with and without drug treatment (lipid-lowering drugs). There was no significant difference in antiarrhythmic treatment.
Table 1.
Characteristics of surgical patients.
| Patient characteristics | NSR | CAF |
|---|---|---|
| Patients, n | 46 | 39 |
| Male/Female | 20/26 | 18/21 |
| Age, years | 40.32±2.10 | 59.40±5.733* |
| Previous history | ||
| CAD, n (%) | 6 (13.04) | 7 (17.94) |
| MVD or AVD, n (%) | 32 (69.56) | 30 (76.92) |
| Hypertension, n (%) | 21 (45.6) | 22 (56.4) |
| Diabetes mellitus, n | 3 (6.52) | 4 (10.25) |
| NYHA functional class, n | ||
| II, n (%) | 29 (63.04) | 5 (12.82)* |
| III, n (%) | 16 (34.78) | 30 (76.9)* |
| IV, n (%) | 1 (2.17) | 4 (10.25)* |
| Echocardiography | ||
| LVEF, % | 56.4±3.1 | 46.6±2.9 |
| LAD, mm | 41.1±2.7 | 54.9±4.1* |
| LVEDD, mm | 52.3±2.4 | 50.2±2.6 |
| IVS | 10.2±0.8 | 8.4±0.7 |
| LVPW | 10.3±0.6 | 9.1±0.5 |
| Medication, n (%) | ||
| Digitalis | 5 (10.86) | 6 (15.38) |
| ACEI/ATRBs | 24 (52.17) | 23 (58.97) |
| β-blockers | 25 (54.34) | 24 (61.53) |
| Diuretics | 13 (28.26) | 15 (38.46) |
| Lipid-lowering drugs | 22 (47.82) | 25 (64.10*) |
| Calcium channels antagonists | 4 (8.69) | 4 (10.25) |
n represents the number of patients, and the percentage represents the percentage of total patients. Data are presented as the mean±SEM.
P<0.05 versus NSR.
CAD – coronary artery disease; MVD – mitral valve disease; AVD – aortic valve disease; LVEF – left ventricular ejection fraction; LAD – left atrial diameter; LVEDD – left ventricular end-diastolic diameter; IVS – interventricular septum thickness; LVPW – left ventricular posterior wall thickness; ACEI – angiotensin-converting enzyme inhibitor. ATRBs, angiotensin receptor blockers. SEM, standard error of the mean.
SK current (IKAS) was increased in atrial myocytes from CAF patients
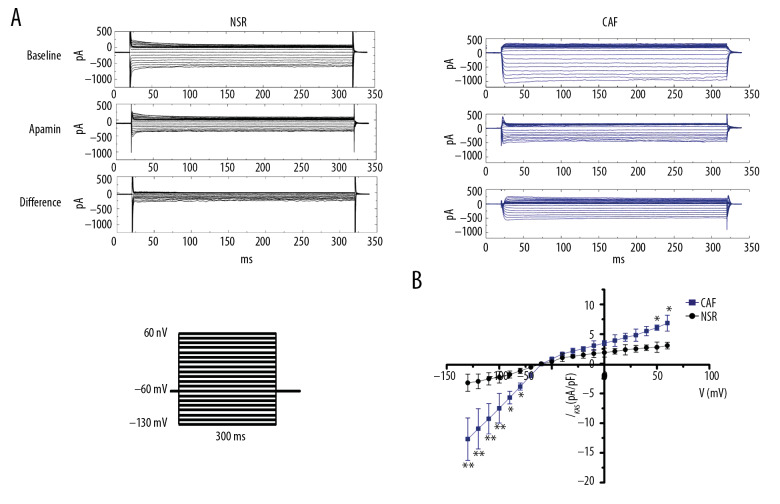
In atrial myocytes from NSR and CAF, whole-cell current was elicited with a holding potential of −60 mV, test potentials ranging from −130 mV to +60 mV for 300 ms. The intracellular free Ca2+ concentration was 500 nmol/L in pipette solution. Figure 1A and 1B show the representative current traces recorded in atrial myocytes in NSR and CAF. IKAS was recorded in the absence and presence of apamin (100 nmol/L, a SK channel selected inhibitor) with intracellular free Ca2+ of 500 nmol/L. Figure 1B shows the current-voltage (I–V) curve of IKAS in atrial myocytes from NSR and CAF (n=15 cells from 8 NSR patients and n=18 cells from 6 CAF patients). The data showed that IKAS was significantly increased in CAF compared with NSR. A striking difference was observed in test potential from −130 mV to −80 mV, and +50 mV and +60 mV.
Figure 1.
IKAS densities of isolated atrial myocytes from patients with normal sinus rhythm (NSR) and chronic atrial fibrillation (CAF). (A) Representative current traces were obtained in atrial myocytes from patients with NSR and CAF. Test pulses ranged from −130mV to +60 mV for 300 ms with a holding potential of −60 mV. The intracellular free Ca2+ was chelated to 500 nmol/L by adding EGTA in pipette solution. In Figure 1A, the difference represented IKAS, which was calculated by subtracting from control to apamin (100 nmol/L). (B) Current-voltage (I–V) relationships of IKAS obtained from NSR and CAF atrial myocytes. * P<0.05, ** P<0.01 versus NSR.
Inhibition of amiodarone on IKAS in patients from NSR and CAF
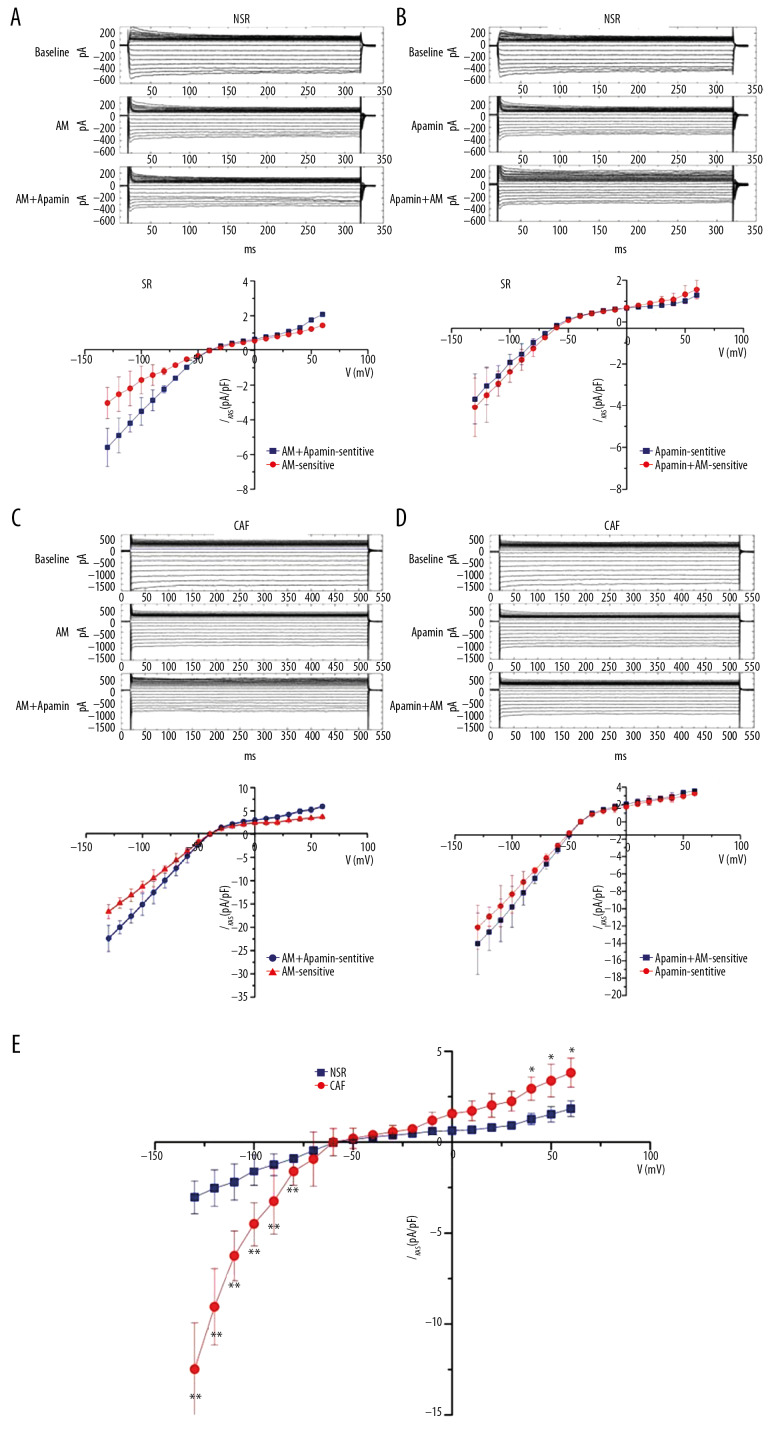
Figure 2 shows the representative recording of amiodarone on IKAS in atrial myocytes from NSR and CAF. The current traces recording in Figure 2A and 2C were performed first to obtain a baseline recording, which was followed by amiodarone 10 μM perfusion for 15 minutes and subsequently amiodarone combined with apamin 100 nM perfusion for 15 minutes. Similarly, the current trace recordings in Figure 2B and 2D were conducted first by obtaining baseline recording, which was followed by perfusion apamin 100 nM for 15 minutes, and then perfused apamin with amiodarone 10 μM for 15 minutes. The data in Figure 2A and 2C reveal that amiodarone apparently inhibited IKAS both in NSR group and CAF group. Nevertheless, after treatment with amiodarone, apamin also inhibited IKAS in both NSR and CAF. This implies that amiodarone partly inhibits IKAS. The data in Figure 2B and 2D unveiled that amiodarone hardly acted on the whole-cell current due to IKAS being fully inhibited by apamin. Furthermore, Figure 2E revealed that the efficacy of amiodarone at −110 mV was stronger in the CAF group (−6.25±1.37 pA/pF, n=8, ** P<0.01) compared to the NSR group (−2.19±0.72 pA/pF, n=9).
Figure 2.
Effect of amiodarone (AM) on IKAS in atrial myocytes from patients with normal sinus rhythm (NSR) and chronic atrial fibrillation (CAF). (A, C) Top: representative current traces recording at baseline and in the presence of AM or AM with apamin in NSR (A) and CAF (C). Bottom: current-voltage (I–V) relation curves of AM-sensitive IKAS (n=12) and AM+apamin-sensitive IKAS (n=9). (B, D) Top: representative current traces recording at baseline and in the presence of apamin or apamin with AM in NSR (B) and CAF (D). Bottom: current-voltage (I–V) relation curves of apamin-sensitive IKAS (n=8) and apamin+AM-sensitive IKAS (n=10). (E) Current-voltage relation curves of IKAS inhibition by AM in NSR (n=8) and CAF (9). * P<0.05, ** P<0.01 versus NSR.
Inhibition of dronedarone on IKAS in atrial myocytes from both NSR and CAF patients
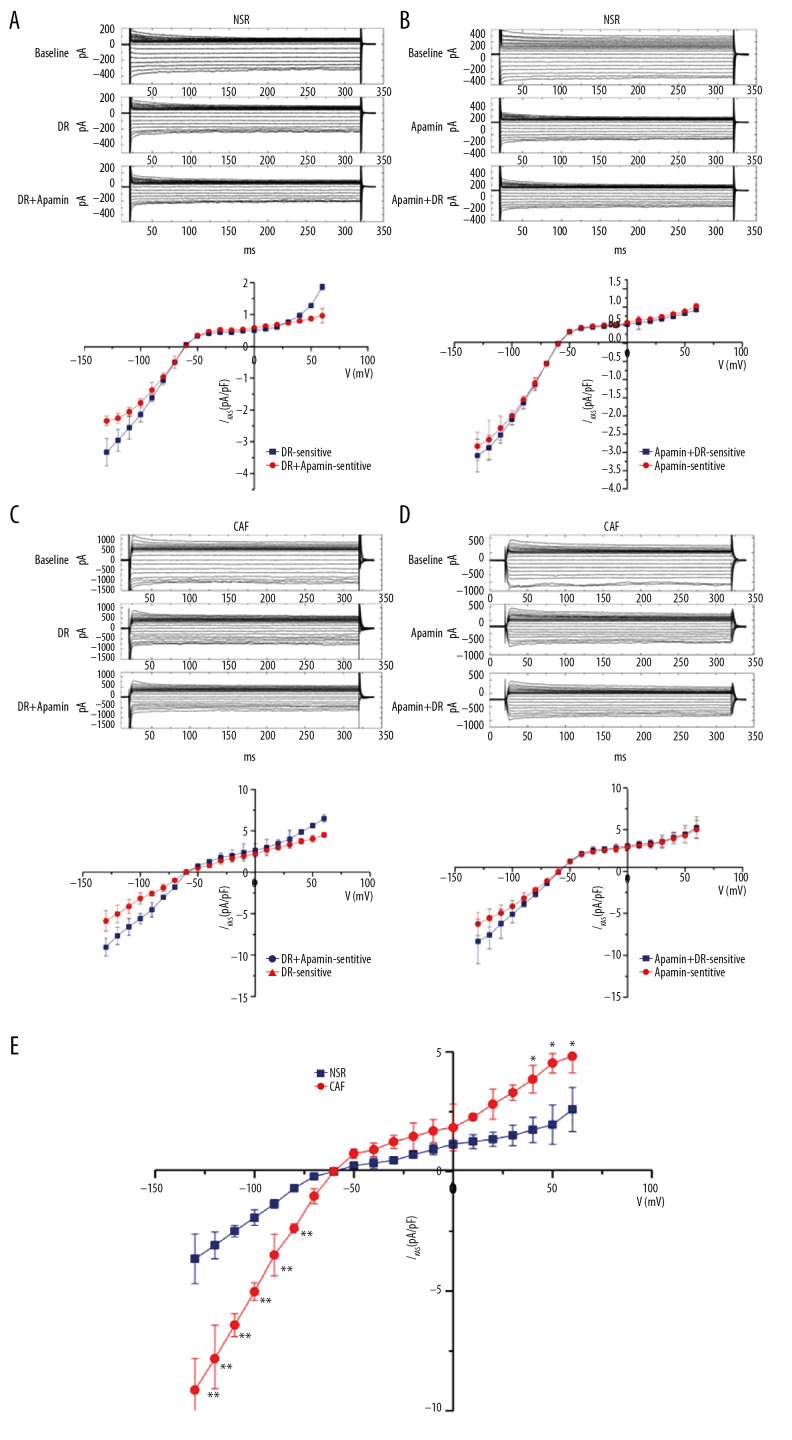
Using the same protocol as amiodarone, the statistical data in Figure 3A and 3C show that dronedarone obviously inhibited IKAS both in the NSR group and the CAF group. Similar to amiodarone, dronedarone 10 μM also could not completely suppress IKAS in atrial myocytes from NSR and CAF patients. This implies that dronedarone also partially suppresses IKAS in the NSR group and the CAF group. Figure 3B and 3D further demonstrate that dronedarone had no effect on the whole-cell current after apamin inhibition. These results confirmed the effects that dronedarone inhibited IKAS in atrial myocytes of NSR and CAF. Furthermore, dronedarone more potently inhibited IKAS at −110 mV in CAF (−7.81±1.49 pA/pF, n=6, ** P<0.01) compared to NSR (−2.47±0.51 pA/pF, n=8) in Figure 3E. This implies that dronedarone could suppress the additional increased IKAS during AF.
Figure 3.
Effect of dronedarone (DR) on IKAS in atrial myocytes from patients with normal sinus rhythm (NSR) and chronic atrial fibrillation (CAF). (A, C) Top: representative currents traces recording at baseline and in the presence of DR or DR with apamin in NSR (A) and CAF (C). Bottom: Current-voltage (I–V) relation curves of DR-sensitive IKAS (n=11) and DR+apamin-sensitive IKAS (n=10). (B, D) Top: representative currents traces recording at baseline and in the presence of apamin or apamin with DR in NSR (B) and CAF (D). Bottom: Current-voltage (I–V) relation curves of apamin-sensitive IKAS (n=8) and apamin+DR-sensitive IKAS (n=10). (E) Current-voltage relation curves of IKAS inhibition by DR in NSR (n=8) and CAF (n=6). * P<0.05, ** P<0.01 versus NSR.
Comparison of the effects between amiodarone and dronedarone on IKAS in human atrial myocytes
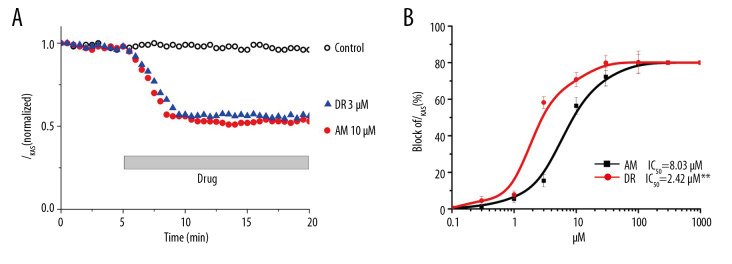
As seen in Figure 4A, amiodarone and dronedarone exhibit a time-dependent inhibition of IKAS. It seems that amiodarone or dronedarone could maintain a stable suppressive effect for almost 10 minutes. In all the patch clamp recordings in atrial myocytes, 15-minute was sufficient to achieve a steady and maximal effect. Figure 4A also indicated that dronedarone was stronger than amiodarone at inhibiting IKAS due to the similar magnitude between 3 μM dronedarone and 10 μM amiodarone on IKAS. The maximum inhibited effect of amiodarone on IKAS (−110 mV) in NSR group was approximately 78.4±3.79% and approximately 81.29±4.31% (n=7, P>0.05) in the CAF group. This indicates no remarkable difference in maximum suppression between SR and AF. The dronedarone data suggested that dronedarone achieved the maximum inhibited effect on IKAS (−110 mV) in the NSR group (82.1±2.93%, n=9) and the CAF group (84.03±5.27%, n=7, P>0.05). There was also no remarkable difference in the maximum suppression effect between NSR and CAF. Unlike the dose of amiodarone used to achieve the maximum suppressed percentage, the same amount of dronedarone resulted in no obvious difference in either the NSR group (n=4, P>0.05) or in the CAF group (n=6, P>0.05). Amiodarone and dronedarone led to a dose-dependent effect (0.3, 1, 3, 10, 30, 100 μM) on IKAS inhibition (Figure 4B). The dose-response curve showed that the IC50 (half maximal inhibitory concentration) value of dronedarone (2.42 μM) for IKAS was lower than amiodarone (8.03 μM) in AF cells. This implies that dronedarone inhibition of IKAS was stronger than that of amiodarone.
Figure 4.
Dose-dependent inhibition of amiodarone and dronedarone on IKAS in atrial myocytes from chronic atrial fibrillation (CAF) patients. (A) Time course of effects on IKAS in atrial myocytes from CAF patients. Baseline keep for 5 minutes, and then perfusion with amiodarone (AM) or dronedarone (DR) for 15 minutes. (B) Dose-response curves of the inhibitory effect of AM and DR on IKAS at −110 mV in atrial myocytes from CAF patients.
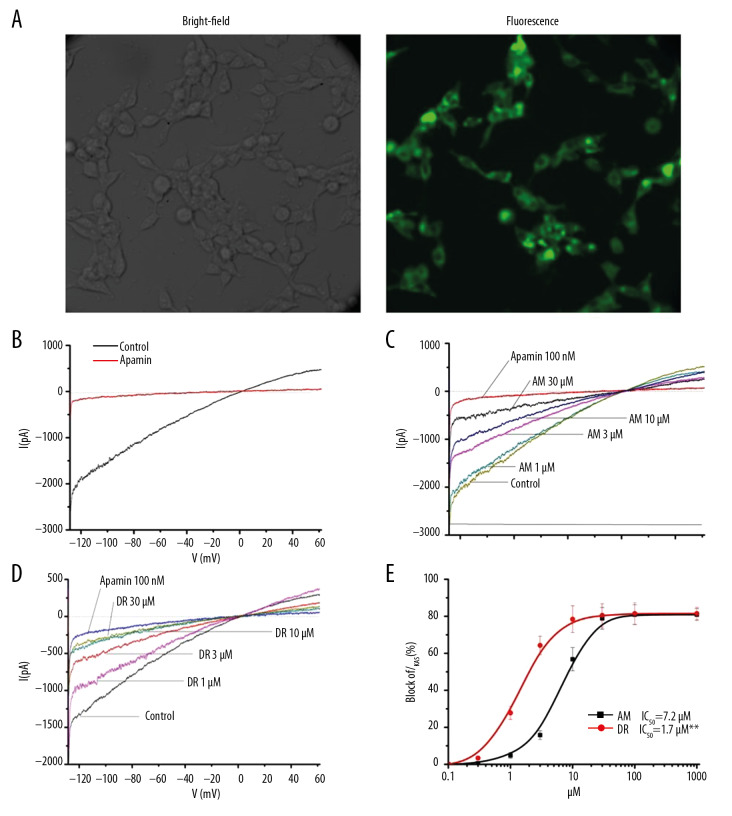
The inhibitory effects of amiodarone and dronedarone on IKAS in HEK-293 cells transfected with KCNN2
In HEK-293 cells, currents were recorded over 48 hours until transient transfection. To produce a larger current at mild negatively membrane potentials, symmetrical high K+ (extracellular and intracellular) solutions were used to current recording with 1 μM intracellular [Ca2+]free [30]. IKAS was identified with 100 nM apamin in transfected KCNN2 in HEK-293 cells. The representative current traces are shown in Figure 5A. The data revealed that apamin significantly blocked IKAS in the HEK-293 cells transfected with KCNN2 (n=8). To measure the pharmacological effects of amiodarone and dronedarone on IKAS in HEK-293. Dose-dependent efficacy is displayed in Figure 5B and 5C. Inhibitory percentages were calculated by the rate values from amiodarone-sensitive to apamin-sensitive. The data showed that amiodarone inhibited IKAS in a concentration-dependent manner (Figure 5D). The maximal suppressive effect was 80.36±3.12% by amiodarone in HEK-293 cells. Similar to amiodarone, dronedarone also produced a dose-dependent manner on IKAS inhibition. Data (−110 mV) were fitted by Hill equation. IC50 value was 1.7 μM for treatment with dronedarone and 7.2 μM for treatment with amiodarone (Figure 5E). There was a significant difference between amiodarone (n=8) and dronedarone (n=8, P<0.01). Therefore, dronedarone clearly had more potent effects on IKAS in HEK-293 cells than amiodarone.
Figure 5.
Inhibition of amiodarone (AM) and dronedarone (DR) on IKAS in HEK-293 cells. (A) GFP expression shows successfully transfected by the SK2 gene. (B) Current traces evoked by a ramp protocol from −130 mV to +60 mV in symmetrical K+ solution. Current recording in the absence and presence 100 nM apamin. (C, D) Current traces of various concentrations of amiodarone or dronedarone, respectively. (E) Dose-response curves of the inhibitory effect of AM (n=8) and DR (n=8) on IKAS at −110 mV in HEK-293 cells.
Discussion
In this study, we investigated the pharmacological inhibitory action of amiodarone and dronedarone on apamin-sensitive small conductance calcium activated potassium channels in human atrial myocytes and HEK-293 cells. The results showed that SK channels were increased in atrial myocytes from CAF patients compared to controls (NSR patients). Amiodarone and dronedarone are class III antiarrhythmic agents that are frequently used in the clinic. Our data showed that these 2 agents produced inhibitory effects on IKAS in a dose-dependent manner in the NSR group and the CAF group. Furthermore, the inhibitory efficacy was stronger in the CAF group than it was in the NSR group. Nevertheless, amiodarone and dronedarone only partly suppress IKAS. The maximum inhibitory efficacy was about 80%. Dronedarone exhibited a more pronounced effect on IKAS than amiodarone in atrial myocytes or HEK-293 cells. These results suggest that amiodarone and dronedarone display antiarrhythmic effects that may be involved in SK channel inhibition.
SK channels and AF
Antiarrhythmic drug therapy is a major management strategy for patients with AF. Compared to no drug therapy, antiarrhythmic therapy is approximately twice as the efficacy in sinus rhythm maintaining [31]. In general, the safety of antiarrhythmic drug therapy is the first factor to be considered. Atrial selective antiarrhythmic agents confer effective AF therapy with few ventricular side-effects. SK channels are more abundant in the atria than in the ventricle, which imparts the important functional difference between the atrial and the ventricle [32]. Recently, a number of studies substantiated that SK channels are closely related with AF development in AF patients and animal AF models [18,19,33,34]. Ozgen et al. observed that burst pacing from pulmonary vein-atria interface induced APD shortening and IKAS increases through SK2 channel proteins trafficking to the cell membrane [35]. In a canine atrial tachypacing model, SK channels were increased in atrial and pulmonary veins. Furthermore, SK channel inhibition alleviated AF susceptibility [22]. Gene knock-out of SK2 also directly demonstrated that SK channels were involved in atrial APD prolongation and early afterdepolarizations [16]. Our previous research also found CaMKII activity was involved in SK current increase during AF [19]. Moreover, some studies revealed that pharmacological inhibition of SK channels was beneficial for AF management [17,33]. The suppression of SK channels for AF treatment is a relatively new treatment. Nevertheless, SK channel inhibition has not been recommended by guidelines for the treatment of atrial fibrillation by European Society of Cardiology (ESC). One of the crucial reasons may be insufficient to understand regarding the mechanism of AF treatment by SK channel inhibition. In fact, it is unclear whether classical antiarrhythmic agents, such as amiodarone or dronedarone, confer anti-AF efficacy partly by targeting on SK channels. Furthermore, it is also necessary to search new agents that target on SK channels.
SK channels inhibited by amiodarone and dronedarone
In this study, we demonstrated that treatment with amiodarone or dronedarone exhibited an inhibitory effect on SK channels in both human atrial myocytes and HEK-293 cells. Our results are similar to the study of Turker et al. [27]. Their study indicated that amiodarone blocked apamin-sensitive potassium currents in a dose-dependent manner that was also Ca2+-dependent. Furthermore, the metabolite of amiodarone (desethylamiodarone) also suppressed IKAS in a voltage-independent and Ca2+-dependent patterns. Interestingly, Kirchhoff et al. found that SK channel inhibitor ICA in combined with amiodarone provided synergistic AF suppression effects [36]. Furthermore, combination of SK blocker and amiodarone conferred beneficial effects not only as an anti-AF treatment but also in terms of alleviating side-effects. Although it has not been directly demonstrated that amiodarone inhibits SK channels, the results support that SK channel blockers with amiodarone contribute to AF prevention. However, Bentzen et al. [26] showed that only dofetilide and propafenone, but not amiodarone or dronedarone, exhibited inhibitory effects on HEK-293 cells transfected with hKCa2.3. Moreover, they also showed that treatment with amiodarone or dronedarone had no significant impact on hKCa2.2. One of the probable explanations taken from our study might be that we evoked IKAS using the conventional patch clamp whole-cell technique with a higher intracellular free Ca2+ concentration (1 μM versus 0.4 μM). In general, manual operated patch clamp is the gold standard for membrane current recording. Bentzen et al. [26] used an automatic patch clamp to record the current. We speculate that while this is not the main cause, it may be a possible factor behind the differences observed. In addition, we measured the inhibitory effects on IKAS not only in HEK-293 cells but also in human atrial myocytes. The effects obtained from human tissues could more veritably reflect the physiological, especially pathological responses.
Amiodarone is one of the most widely documented antiarrhythmic drugs for AF treatment. Extracardiac side-effects limit its widespread usage in clinic. Dronedarone synthetic exhibits a similar antiarrhythmic efficacy in paroxysmal or persistent AF with fewer side effects. In this study, we found that dronedarone produced a stronger efficacy than amiodarone on SK channel inhibition in both HEK-293 cells and atrial myocytes from patients. The IC50 of dronedarone is approximately 3 to 4 time lower than that of amiodarone at −110 mV (2.43 μM versus 8.03 μM). It implies that dronedarone and amiodarone confer SK channel inhibition in a clinical effective concentration. Furthermore, the inhibitory effects of dronedarone and amiodarone were stronger in CAF compared to NSR. This data appears to indicate that this efficacy is advantageous for AF treatment due to a decrease in the excess IKAS during AF. These results are consistent with the study for relieving AF by SK channel suppression. Interestingly, in our study, the maximum inhibitory efficacy both in dronedarone and amiodarone was approximately 80% either in NSR or in CAF, either in atrial myocytes or in HEK-293 cells, even in a high concentration (higher than 30 μM). This result is similar to the result reported by Turker et al. (85.6%). Actually, studies have reported that dronedarone leads to approximately 50% of membrane ion channels with a maximum inhibitory effect approaching 100% [37,38]. Half of channels being only partially blocked by dronedarone. Complete suppression might be a risk factor for pro-arrhythmia. Our study demonstrated that treatment with amiodarone or dronedarone can partially inhibit SK channels.
Clinical value of SK channel inhibition
Ideally, atrial-selected drugs can directly target the channels mainly in the atria during atrial arrhythmia, and they have no cardiac or extracardiac adverse effects [39,40]. SK channels belong to this type of channel and they are preferentially expressed in the atria rather than the ventricle. Several studies, including those of our group, have demonstrated SK channels increased during AF or atrial rapid pacing animal models. Although some studies reported that SK channels have no significant contribution to atrial repolarization [41–43], more studies supported that SK channel inhibition produces efficacy for the prevention of AF development [44,45]. Until now, few drugs have been demonstrated to target SK channels in clinical practices. In fact, many effective antiarrhythmic drugs preferentially target multiply channels, such as amiodarone or dronedarone. In this study, we demonstrated that amiodarone and dronedarone both target SK channels. This implies that effective antiarrhythmic drugs for AF treatment partly rely on SK channel inhibition. In addition to systematically testing the known antiarrhythmic drugs, searching for new drugs that target SK channels might be a new strategy for identifying ways to manage AF.
Limitations in this study
There were several limitations in this study. First, we tested amiodarone and dronedarone on SK channels. It would be more convincing if we test additional class III antiarrhythmic drugs. Second, SK2 (KCNN2) is a predominant channel subunit in cardiac myocytes. therefore, we measured the effects on SK2 channel in HEK-293 cells. Other SK channel subunits such as KCNN1 and KCNN3, were not tested in this study. It is difficult to identify the electrophysiology profiles of channel subunits in cardiac myocytes. Third, SK channels are sensitive to free intracellular Ca2+. Although we did not measure the variance of intracellular free Ca2+, the intracellular free Ca2+ in atrial myocytes was 0.5 μM and it was 1 μM in HEK-293 cells, which is sufficient to activate SK channels.
Conclusions
Amiodarone and dronedarone both inhibit SK channels in human atrial myocytes and HEK-293 cells. The potent suppression of SK channels may represent a potential therapeutic target for AF treatment. Dronedarone was more effective than amiodarone in SK channel inhibition., which implies that dronedarone has fewer side effects and is superior to amiodarone on SK channel inhibition.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81470022) and Department of Science and Technology of Sichuan Province Grant (19YYJC1958)
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–62. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Andrade JG, Macle L, Nattel S, et al. Contemporary atrial fibrillation management: A comparison of the current AHA/ACC/HRS, CCS, and ESC Guidelines. Can J Cardiol. 2017;33(8):965–76. doi: 10.1016/j.cjca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Akao M, Chun YH, Wada H, et al. Current status of clinical background of patients with atrial fibrillation in a community-based survey: The Fushimi AF Registry. J Cardiol. 2013;61(4):260–66. doi: 10.1016/j.jjcc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the early treatment of atrial fibrillation for stroke prevention trial. Am Heart J. 2013;166(3):442–48. doi: 10.1016/j.ahj.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261–74. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs. medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321(13):1275–85. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burashnikov A, Di Diego JM, Sicouri S, et al. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5(12):1735–42. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129(5):468–75. doi: 10.1016/j.amjmed.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4(9):1250–59. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Moroi MK, Ruzieh M, Aboujamous NM, et al. Dataset for amiodarone adverse events compared to placebo using data from randomized controlled trials. Data Brief. 2020;28:104835. doi: 10.1016/j.dib.2019.104835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terzo F, Ricci A, D’Ascanio M, et al. Amiodarone-induced pulmonary toxicity with an excellent response to treatment: A case report. Respir Med Case Rep. 2020;29:100974. doi: 10.1016/j.rmcr.2019.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer PA, Becker R, Katus HA, et al. Dronedarone: Current evidence for its safety and efficacy in the management of atrial fibrillation. Drug Des Devel Ther. 2011;5:27–39. doi: 10.2147/DDDT.S10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer JA, Kjesbo NK, Gleason PP. Dronedarone: Current evidence and future questions. Cardiovasc Ther. 2010;8(1):38–47. doi: 10.1111/j.1755-5922.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 14.Altomare C, Barbuti A, Viscomi C, et al. Effects of dronedarone on acetylcholine-activated current in rabbit SAN cells. Br J Pharmacol. 2000;130(6):1315–20. doi: 10.1038/sj.bjp.0703446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burashnikov A, Belardinelli L, Antzelevitch C. Acute dronedarone is inferior to amiodarone in terminating and preventing atrial fibrillation in canine atria. Heart Rhythm. 2010;7(9):1273–79. doi: 10.1016/j.hrthm.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Timofeyev V, Tuteja D, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587(Pt 5):1087–100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skibsbye L, Bengaard AK, Uldum-Nielsen AM, et al. Inhibition of small conductance calcium-activated potassium (SK) channels prevents arrhythmias in rat atria during beta-adrenergic and muscarinic receptor activation. Front Physiol. 2018;9:510. doi: 10.3389/fphys.2018.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li ML, Li T, Lei M, et al. [Increased small conductance calcium-activated potassium channel (SK2 channel) current in atrial myocytes of patients with persistent atrial fibrillation]. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(2):147–51. [in Chinese] [PubMed] [Google Scholar]
- 19.Fan X, Yu Y, Lan H, et al. Ca2+/calmodulin-dependent protein kinase II (CaMKII) increases small-conductance Ca2+-activated K+ current in patients with chronic atrial fibrillation. Med Sci Monit. 2018;24:3011–23. doi: 10.12659/MSM.909684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diness JG, Skibsbye L, Simo-Vicens R, et al. Termination of Vernakalant-resistant atrial fibrillation by inhibition of small-conductance Ca(2+)-activated K(+) channels in pigs. Circ Arrhythm Electrophysiol. 2017;10(10) doi: 10.1161/CIRCEP.117.005125. pii: e005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diness JG, Kirchhoff JE, Sheykhzade M, et al. Antiarrhythmic effect of either negative modulation or blockade of small conductance Ca2+-activated K+ channels on ventricular fibrillation in guinea pig Langendorff-perfused heart. J Cardiovasc Pharmacol. 2015;66(3):294–99. doi: 10.1097/FJC.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 22.Qi XY, Diness JG, Brundel BJ, et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129(4):430–40. doi: 10.1161/CIRCULATIONAHA.113.003019. [DOI] [PubMed] [Google Scholar]
- 23.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): Rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52(2):105–20. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 24.Dobrev D, Friedrich A, Voigt N, et al. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112(24):3697–706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 25.Heijman J, Dobrev D. Inhibition of small-conductance Ca(2+)-activated K(+) channels: the long-awaited breakthrough for antiarrhythmic drug therapy of atrial fibrillation? Circ Arrhythm Electrophysiol. 2017;10(10) doi: 10.1161/CIRCEP.117.005776. pii: e005776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simo-Vicens R, Sauter DRP, Grunnet, et al. Effect of antiarrhythmic drugs on small conductance calcium – activated potassium channels. Eur J Pharmacol. 2017;803:118–23. doi: 10.1016/j.ejphar.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Turker I, Yu CC, Chang PC, et al. Amiodarone inhibits apamin-sensitive potassium currents. PLoS One. 2013;8(7):e70450. doi: 10.1371/journal.pone.0070450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch RF, Zeng X, Grammer JB, et al. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44(1):121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 29.Narayana SK, Woods DR, Boos CJ. Management of amiodarone-related thyroid problems. Ther Adv Endocrinol Metab. 2011;2(3):115–26. doi: 10.1177/2042018811398516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamy C, Scuvee-Moreau J, Dilly S, et al. The sigma agonist 1,3-di-o-tolyl-guanidine directly blocks SK channels in dopaminergic neurons and in cell lines. Eur J Pharmacol. 2010;641(1):23–28. doi: 10.1016/j.ejphar.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278(49):49085–94. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 33.Diness JG, Bentzen BH, Sorensen US, et al. Role of calcium-activated potassium channels in atrial fibrillation pathophysiology and therapy. J Cardiovasc Pharmacol. 2015;66(5):441–48. doi: 10.1097/FJC.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haugaard MM, Hesselkilde EZ, Pehrson S, et al. Pharmacologic inhibition of small-conductance calcium-activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart Rhythm. 2015;12(4):825–35. doi: 10.1016/j.hrthm.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Ozgen N, Dun W, Sosunov EA, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75(4):758–69. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchhoff JE, Diness JG, Abildgaard L, et al. Antiarrhythmic effect of the Ca(2+)-activated K(+) (SK) channel inhibitor ICA combined with either amiodarone or dofetilide in an isolated heart model of atrial fibrillation. Pflugers Arch. 2016;468(11–12):1853–63. doi: 10.1007/s00424-016-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt C, Wiedmann F, Schweizer PA, et al. Novel electrophysiological properties of dronedarone: Inhibition of human cardiac two-pore-domain potassium (K2P) channels. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(10):1003–16. doi: 10.1007/s00210-012-0780-9. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann N, Mason FE, Braun I, et al. The combined effects of ranolazine and dronedarone on human atrial and ventricular electrophysiology. J Mol Cell Cardiol. 2016;94:95–106. doi: 10.1016/j.yjmcc.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Ravens U, Odening KE. Atrial fibrillation: Therapeutic potential of atrial K(+) channel blockers. Pharmacol Ther. 2017;176:13–21. doi: 10.1016/j.pharmthera.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Ravens U. Atrial-selective K(+) channel blockers: Potential antiarrhythmic drugs in atrial fibrillation? Can J Physiol Pharmacol. 2017;95(11):1313–18. doi: 10.1139/cjpp-2017-0024. [DOI] [PubMed] [Google Scholar]
- 41.Bonilla IM, Long VP, 3rd, Vargas-Pinto P, et al. Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PLoS One. 2014;9(10):e108824. doi: 10.1371/journal.pone.0108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy N, Szuts V, Horvath Z, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009;47(5):656–63. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Yu T, Deng C, Wu R, et al. Decreased expression of small-conductance Ca2+-activated K+ channels SK1 and SK2 in human chronic atrial fibrillation. Life Sci. 2012;90(5–6):219–27. doi: 10.1016/j.lfs.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh YC, Chang PC, Hsueh CH, et al. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol. 2013;6(2):410–18. doi: 10.1161/CIRCEP.111.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terentyev D, Rochira JA, Terentyeva R, et al. Sarcoplasmic reticulum Ca(2)(+) release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2014;306(5):H738–46. doi: 10.1152/ajpheart.00621.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]