Fig 1. Folded and fibrillar states.
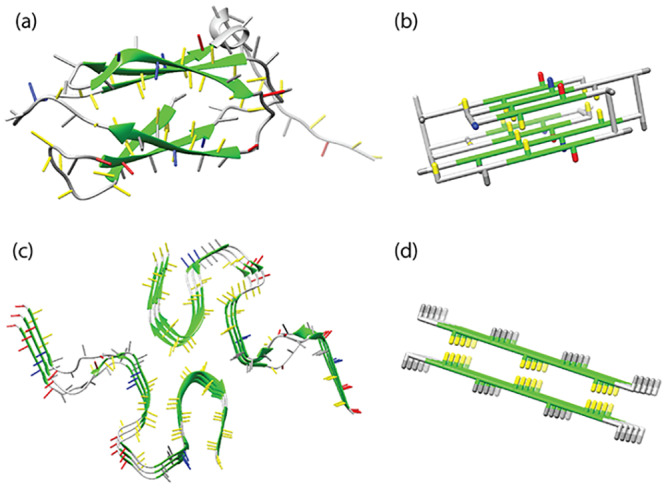
(A) The native state of human transglutaminase (PDB-ID: 2XZZ) (B) a model protein in the native state, (C) disease-related amyloid β-sheet of the Aβ (1-42) peptide (PDB-ID: 2NAO) [28] and (D) the seed structure of an amyloid fibril represented in the lattice model used for this work. Side chains of hydrophobic residues are coloured in yellow, of polar residues in grey, of positively charged residues in blue and of negatively charged residues in red. For β-stranded structures the backbone is coloured in green. In the folded protein, a hydrophobic core can be observed, where the hydrophobic residues are shielded from the water by the hydrophilic and charged residues. Similarly, the core sequence regions of amyloid fibril structures tend to be strongly hydrophobic.
