Figure 7.
Ser784 Phosphorylation of VCP Is Important for DNA-Damage Response and Cell Survival upon Genotoxic Stress
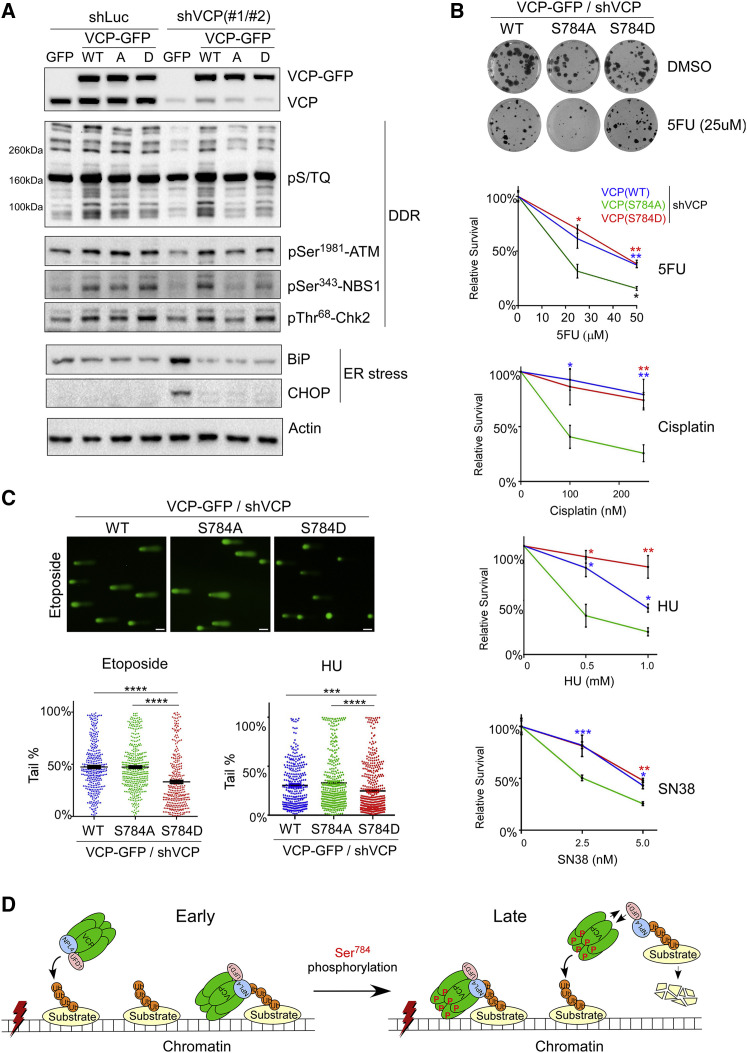
(A) shLuc- or shVCP-infected stable HeLa cells were treated with 50 μM of etoposide for 30 min, recovered for 1 h, lysed by RIPA, and analyzed by western blot.
(B) shLuc- or shVCP-infected stable U2OS cells were treated with vehicle or different drugs for 16 h, and subjected to colony formation assays for 10–14 days (Guzmán et al., 2014). Relative effects represent normalized drug/vehicle percentages. Shown are means ± SEM of three technical replicates of single biological experiments for each drug. p Values were based on unpaired t tests (S784A versus WT or S784D).
(C) shVCP2-infected U2OS stable cells were treated with 25 μM of etoposide for 30 min followed by 1 h of recovery or 1 mM HU for 16 h. Cells were subjected to the comet assay under the alkaline condition, and tail DNA percentages were calculated (Gyori et al., 2014). Shown are single biological experiments with 150–300 cells analyzed per condition. Error bars represent SEM. p Values were based on unpaired t tests. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Results in (B) and (C) were confirmed by three biological replicates.
(D) Working model of enhanced substrate extraction from chromatin by VCP upon DNA-damage-induced Ser784 phosphorylation. In this model, Ser784 phosphorylation is a relatively late DDR event that occurs either in the nucleoplasm or on chromatin after VCP binding to polyubiquitinated substrates (both scenarios are depicted). Ser784 phosphorylation does not abolish chromatin recruitment of VCP but promotes substrate extraction and subsequent degradation at least partially because of its weakened interaction with cofactors NPL4 and UFD1, which directly bind substrates. Dissociated pSer784-VCP can regain access to chromatin and extract more substrates.
Scale bars, 40 μm. See also Figures S9 and S10.