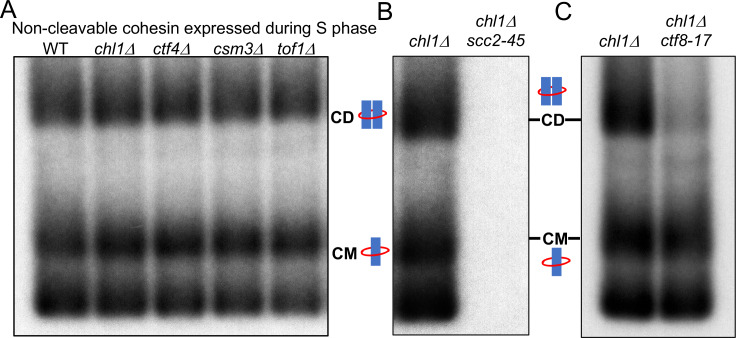
Figure 5. Cohesion can also be established by CTF18-RFC and Scc2 dependent de novo loading of nucleoplasmic cohesin.
(A) Wild type (K24697), chl1Δ (K28175), ctf4Δ (K28275), csm3Δ (K28282) and tof1Δ (K28280) strains that contain genes coding for 6C non cleavable cohesin (2C SMC1 2C SMC3 and GALp-2C SCC1NC) were arrested in G1 phase, the expression of 2C Scc1NC was induced by addition of galactose for 15 min. The cultures were released from the G1 arrest into medium containing galactose in order to ensure constant expression of Scc1NC during DNA replication, mini-chromosome IP of the cultures after replication shows CM and CD formation in all the strains. Data shown is representative of two independent biological repeats. (B) chl1Δ (K28175) and chl1Δ scc2-45 (K28061) strains that contain genes coding for 6C non-cleavable cohesin (2C SMC1 2C SMC3 and GALp-2C SCC1NC) were arrested in G1 phase, the expression of 2C Scc1NC was induced by addition of galactose for 15 min. The cultures were released from the G1 arrest into medium containing galactose in order to ensure constant expression of Scc1NC during DNA replication at 37°C (in order to inactivate Scc2 in the scc2-45 strain). Mini-chromosome IP of the cultures after replication shows CM and CD formation in the chl1Δ while in the chl1Δ scc2-45 we failed to IP any mini-chromosome DNA. Data shown is representative of two independent biological repeats. (C) chl1Δ (K28175) and chl1Δ ctf8-17 (K28295) strains that contain genes coding for 6C non cleavable cohesin (2C SMC1 2C SMC3 and GALp-2C SCC1NC) were arrested in G1 phase, the expression of 2C Scc1NC was induced by addition of galactose for 15 min. The cultures were released from the G1 arrest into medium containing galactose in order to ensure constant expression of Scc1NC during DNA replication at 37°C (in order to inactivate Ctf8 in the ctf8-17 strain). Mini-chromosome IP of the cultures after replication shows CM and CD formation in the chl1Δ. While the chl1Δ ctf8-17 strains showed the presence of CMs, the level of CDs was greatly reduced. Data shown is representative of two independent biological repeats.