Abstract
Background
A Pediatric Brain Tumor Consortium (PBTC) phase I/II trial of veliparib and radiation followed by veliparib and temozolomide (TMZ) was conducted in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). The objectives were to: (i) estimate the recommended phase II dose (RP2D) of veliparib with concurrent radiation; (ii) evaluate the pharmacokinetic parameters of veliparib during radiation; (iii) evaluate feasibility of intrapatient TMZ dose escalation; (iv) describe toxicities of protocol therapy; and (v) estimate the overall survival distribution compared with historical series.
Methods
Veliparib was given Monday through Friday b.i.d. during radiation followed by a 4-week rest. Patients then received veliparib at 25 mg/m2 b.i.d. and TMZ 135 mg/m2 daily for 5 days every 28 days. Intrapatient dose escalation of TMZ was investigated for patients experiencing minimal toxicity.
Results
Sixty-six patients (65 eligible) were enrolled. The RP2D of veliparib was 65 mg/m2 b.i.d. with radiation. Dose-limiting toxicities during radiation with veliparib therapy included: grade 2 intratumoral hemorrhage (n = 1), grade 3 maculopapular rash (n = 2), and grade 3 nervous system disorder (generalized neurologic deterioration) (n = 1). Intrapatient TMZ dose escalation during maintenance was not tolerated. Following a planned interim analysis, it was concluded that this treatment did not show a survival benefit compared with PBTC historical controls, and accrual was stopped for futility. The 1- and 2-year overall survival rates were 37.2% (SE 7%) and 5.3% (SE 3%), respectively.
Conclusion
Addition of veliparib to radiation followed by TMZ and veliparib was tolerated but did not improve survival for patients with newly diagnosed DIPG.
Trial Registration
Keywords: ABT-888, CNS tumors, DIPG, PARP inhibition, veliparib
Key Points.
1. Veliparib and radiation followed by maintenance therapy with TMZ and veliparib was safe and well tolerated in pediatric DIPG patients.
2. This therapy did not offer prolonged survival compared with historical controls.
Importance of the Study.
The prognosis for patients with DIPG remains dismal, highlighting the urgent need for new therapies. This trial established the recommended phase II dose of veliparib combined with radiation therapy in newly diagnosed patients with DIPG. Following radiation, the combination of veliparib plus TMZ was tolerable at previously established doses.1 While tolerable, this approach did not improve survival in patients with DIPG; however, it may warrant further evaluation in other patient populations, such as high-grade gliomas.
Despite decades of clinical trials for patients with diffuse intrinsic pontine glioma (DIPG), outcomes remain poor, with radiation therapy remaining standard of care and survival rates less than 10% at 2 years.2,3 DIPG is historically a radiographic diagnosis; however, recent studies demonstrate the safety of biopsy,4 and tissues collected at surgery and autopsy have led to molecular classification of tumors, with the majority having H3F3A or HIST1H3B mutations.5–7 In addition, studies demonstrate elevated poly(ADP-ribose) polymerase (PARP) expression and/or activity in pediatric glioblastoma (GBM) and DIPG,8–10 hence identifying PARP as a novel therapeutic target. The family of PARP proteins repair single-stranded DNA breaks through base excision repair and are involved in double-stranded break repair through chromatin remodeling and regulation of nonhomologous end-joining (NHEJ) and homologous recombination repair (HRR).11–13 Therefore, PARP inhibition has been developed as a novel strategy to inhibit DNA repairs and enhance chemotherapy and radiation sensitivity.14,15
Veliparib, an oral PARP inhibitor, has been shown to enhance the preclinical anticancer potential of multiple chemotherapy agents, including temozolomide (TMZ),16,17 and enhance radiation efficacy18,19 and has excellent CNS entry.20 In a phase I trial of veliparib and TMZ in children with recurrent CNS tumors conducted through the PBTC, we established the RP2D of veliparib 25 mg/m2 b.i.d. and TMZ 135 mg/m2 daily, given 5 days every 28 days. We observed prolonged stable disease (SD) in a patient with GBM for greater than 6 months and a patient with DIPG who had a 40% reduction in tumor size after one cycle.1
We report results of a PBTC phase I/II trial of veliparib and radiation, followed by veliparib and TMZ, in children with newly diagnosed DIPG. The primary objectives of this trial were to identify the maximum tolerated dose (MTD) or RP2D of veliparib administered concurrently with radiation and to describe the associated toxicities and plasma pharmacokinetic (PK) parameters of veliparib during radiation (phase I component); to study the feasibility of intrapatient dose escalation of TMZ during maintenance therapy; to estimate the number of patients experiencing pseudo progression from therapy (phase I and phase II components); and to estimate the overall survival (OS) distribution and compare with historical controls (phase II component). The secondary objectives were to estimate progression-free survival (PFS); summarize the best response; explore peripheral blood mononuclear cell (PMBC) PARP activity and DNA repair proteins before and after treatment; evaluate PARP activity and DNA repair proteins in biopsied tumors; explore imaging correlatives with disease outcomes; and evaluate urinary biomarkers for detecting and monitoring pediatric gliomas.
Patients and Methods
Eligibility
Children ≤21 years of age with newly diagnosed DIPG, defined as tumors with a pontine epicenter and diffuse intrinsic involvement of the pons, were eligible without biopsy. Patients with atypical tumors were only eligible if a biopsy showed histology consistent with fibrillary astrocytoma, anaplastic astrocytoma, GBM, gliosarcoma, or anaplastic mixed glioma. Patients were required to have a Karnofsky or Lansky performance status ≥50. Patients must not have received prior therapy for their DIPG; had to be able to swallow oral medications; and have adequate baseline renal, hepatic, and hematologic functions. Patients were excluded if they were receiving other anticancer or experimental therapy; had systemic illness, uncontrolled infection, or seizures; or were pregnant or lactating. The institutional review board of each participating site approved the clinical trial. Written informed consent and assent were obtained according to institutional guidelines.
Study Design
Veliparib (ABT-888; NSC# 737664) was supplied by AbbVie and distributed by the National Cancer Institute’s (NCI) Division of Cancer Treatment and Diagnosis as immediate release capsules or a 5 mg/mL suspension. Veliparib was given b.i.d. Monday through Friday during radiation. The morning dose was given 60–120 minutes prior to radiation when possible. The starting dose level of veliparib during radiation was 50 mg/m2/dose b.i.d., approximately 80% of the adult dose of 100 mg b.i.d.,21 with 2 planned dose escalations (65 and 85 mg/m2/dose b.i.d.) and 1 planned de-escalation (35 mg/m2/dose b.i.d.). Patients received a dose of 5400 cGy (centigray) administered in 30 fractions over 6 weeks to the planning target volume in addition to veliparib.
Maintenance therapy began 4 weeks following radiation provided that the patient had fully recovered to meet on study criteria. Starting maintenance therapy were 25 mg/m2/dose b.i.d. of veliparib and TMZ 135 mg/m2/day, for 5 days every 28 days. Starting with maintenance course 2, intrapatient TMZ dose escalation was attempted for patients with predefined minimal toxicities in the preceding course, defined as grade ≤1 thrombocytopenia and grade ≤2 neutropenia and grade ≤2 non-hematologic toxicities. TMZ was escalated to 175 mg/m2/day and 200 mg/m2/day in subsequent courses, as tolerated. Intrapatient dose escalation was permanently stopped for patients experiencing toxicities exceeding predefined threshold in any maintenance course. Initiation of each course of maintenance required absolute neutrophil count ≥1000/mm3 and platelets ≥100 000/mm3. Treatment could continue, in the absence of disease progression or toxicity requiring discontinuation of therapy, for up to 10 courses, with the option for continuing therapy for 13 additional courses.
A traditional 3 + 3 phase I dose escalation was used wherein the MTD was exceeded if either 1 out of 3 or 2 out of 6 subjects experienced dose-limiting toxicities (DLTs) during radiation therapy. The design of the phase II study was based on the Bayesian approach developed by Thall, Wooten, and Tannir,22 where outcome from this study was to be compared with a historical cohort of 140 DIPG patients treated on prior PBTC studies PBTC-006 (phase I imatinib), PBTC-007 (phase I and phase II gefitinib), and PBTC-014 (phase I and phase II tipifarnib). OS was the primary endpoint and defined as the time interval from starting treatment to death from any cause or to date of last follow-up. Failure times were modeled via an exponential distribution with an inverse gamma prior on the mean. The prior was based on the median OS estimate of 10.85 months from the PBTC historical cohort, and the target “effect size” was set as median OS of 16 months with a max sample size of 60 subjects. The trial also incorporated every 6-month monitoring as part of the early stopping assessment. PFS was a secondary endpoint, defined as the time from starting treatment to the earliest date of failure. For patients suspected of having pseudoprogression, defined as tumors with 25% or greater progression by bidimensional MRI area, but improved spontaneously to a size of SD or smaller compared with baseline on subsequent scans, during the first 6 months of therapy, the treating physician had the option of continuing therapy and repeating an evaluation in 4–6 weeks. If the repeat MRI showed true progression, then the date of progression was the initial MRI that suggested progression. OS and PFS distributions were estimated using the method of Kaplan and Meier. The exact log rank test was used to compare outcome distributions among patient groups (eg, poly(ADP-ribose) levels).The Wilcoxon-Mann-Whitney test was used to examine differences in the distributions of age and urine biomarkers among cases and controls. The chi-square test was used to examine differences in gender distributions by group. Logistic regression was used to examine whether biomarkers were predictive for differentiating DIPG versus controls adjusting for age and sex. Receiver operating characteristic curve analysis was done to assess the predictive accuracy of the biomarkers with area under the curve and 95% confidence intervals as indices of performance in differentiating between DIPG and controls. Youden’s index was used to identify optimal cutoff values for the biomarkers of most interest; based on these cutoff values, sensitivity, specificity, and accuracy were calculated. Cox proportional hazards regression was used to examine association of urine biomarkers and imaging parameters at the time of diagnosis with outcome. P-values were two-sided and those less than 0.05 were considered statistically significant.
Monitoring
Toxicity monitoring included weekly physical examinations and laboratory studies (complete blood count, serum chemistries, and renal and hepatic functions) during radiation therapy and prior to each maintenance course. Complete blood counts were also monitored weekly throughout protocol therapy. Disease evaluations with MRI were obtained at baseline and prior to maintenance therapy, and then prior to courses 3, 5, and 8, and at the end of maintenance. If pseudoprogression was suspected at weeks 10, 18, or 26, patients could remain on therapy provided that there was not clinical deterioration consistent with tumor progression, they had been on a stable dose of steroids, and subsequent MRI was stable or improved. Objective responses underwent central MRI review.
Definition of DLT and MTD
Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events v4.0. DLT, defined as any event that was at least possibly, probably, or definitely attributable to veliparib observed during the first 10 weeks of therapy, included: any interruption of irradiation related to protocol therapy for 5 consecutive or 10 cumulative days; any grade 4 non-hematologic toxicity; any grade 2 non-hematologic toxicity persisting for >7 days and was considered medically significant; any grade 3 non-hematologic toxicity (except grade 3 nausea/vomiting <5 days, grade 3 elevation of transaminases that return to on-study levels within 7 days, grade 3 fever or infection for <5 days, grade 3 electrolyte abnormalities responsive to oral supplementation); grade 4 neutropenia; and grade 3 thrombocytopenia.
The MTD was defined as the highest dose level at which no more than 1 out of 6 patients experienced DLTs, and the next higher dose level was deemed too toxic. Patients who received less than 85% of the veliparib doses during radiation therapy for reasons other than toxicity were considered inevaluable for estimating the MTD and were replaced.
Pharmacokinetic Studies
Blood samples were collected pre-veliparib course 1 day 1, at 0.5, 1, 2, and 6–8 hours after the first dose, pre-veliparib on day 4 (steady state), and 2 hours after the morning dose. Samples were collected and processed as previously described and veliparib concentrations were measured by AbbVie using a previously described liquid chromatography–tandem mass spectrometry assay.1 PK parameters were evaluated using a non-compartmental analysis.
PARP Inhibition in Peripheral Blood Monocytes
Blood samples were collected prior to the start of veliparib and radiation, during the third and sixth weeks of radiation, and during the first course of maintenance therapy. Samples were shipped at room temperature and PBMCs were isolated (CPT Cell Preparation Tube with sodium citrate) within 24–36 hours after receipt and stored at −80°C until analysis. An enzyme-linked immunosorbent assay (ELISA) was used to measure poly(ADP-ribose) (PAR) levels in PBMCs.23 Each sample was analyzed by protein-load, performed in duplicates, and quantified as pg/mL.
ELISA Analysis of DNA Repair Proteins in PBMC
PBMCs were lysed by washing in phosphate buffered saline and freezing to −20°C and thawing to room temperature 3 times, centrifuged at 1500 g for 10 minutes at 4°C, and the supernatant was collected for analysis. Protein concentrations were determined using the Bradford protein assay (Bio-Rad #500–0006). The Sandwich ELISA kits from Lifespan Biosciences, Inc (LS-F9958–1 for BRCA2; LS-F4618 for KU70; LS-F8678 for MLH1; LS-F12266 for PARP; LS-F20942 for ATM) were used to measure protein levels following manufacturer’s manual. Each sample was run in duplicate and the optical density values at 450 nm (BioTek Synergy 2) were calculated by subtracting negative control blank.
Immunohistochemical Studies of FFPE Tumor Slides
Immunohistochemical staining was performed on 4-micron, formalin-fixed paraffin embedded (FFPE) tissue sections using standard techniques, with tissue-appropriate controls as previously described.1
Urine Biomarker Studies
Urine samples were collected at enrollment in consenting patients, post-veliparib and radiation, and then at the time of each MRI. Urine was collected, placed on ice, and stored at −20°C until shipped. All samples were assayed for total protein concentration by Bradford analysis (Bio-Rad Laboratories) as previously described.24,25 ELISAs were performed according to manufacturer instructions and read using a FilterMax F3 spectrophotometer (Molecular Devices). Netrin-1 levels were determined by ELISA (US Biomax). Levels of matrix metalloproteinase (MMP)-2, MMP-3, MMP-9, MMP-13, angiogenin, angiopoietin, placental growth factor (PlGF), thrombospondin-2, vascular endothelial growth factor, epidermal growth factor (EGF), hepatocyte growth factor, heparin-binding EGF-like growth factor, and basic fibroblast growth factor (bFGF) were analyzed using custom Luminex screening assays (R&D Systems). Tissue inhibitor of metalloproteinase (TIMP-1), TIMP-2, TIMP-3, and TIMP-4 levels were analyzed using a Luminex performance assay (R&D Systems). All Luminex assays were performed according to manufacturer instructions, and read using a Bio-Plex 200 HTF system (Bio-Rad Laboratories). Protein concentrations are given in picograms per microgram (pg/μg), and determined by dividing the concentration of the target protein in the sample (pg/mL) by the concentration of total protein in the sample (μg/mL).
Neuroimaging
An MRI (sagittal T1, axial fluid attenuated inversion recovery [FLAIR], axial T2, post-gadolinium sagittal T1 with reformats) was obtained prior to registration, at the end of radiation therapy (week 10), within 1 week prior to courses 3, 5, and 8 of maintenance, and at the end of treatment. Dynamic susceptibility contrast MR perfusion and T1 permeability perfusion and diffusion imaging were obtained at the same timepoints through week 26. Apparent diffusion coefficient (ADC) histogram analysis was also obtained. Volumetric analysis was done using a Vitrea workstation from the axial FLAIR and T1-weighted post contrast brain images. A complete response (CR) was defined as complete disappearance of all enhancing tumor and tumor on T2 FLAIR/T2 images, and a partial response (PR) was defined by >50% reduction in tumor size by bidimensional measurements from FLAIR or T2 images, maintained for at least 8 weeks, accompanied by a stable or improving neurologic examination and a stable or decreasing dose of corticosteroids. Progressive disease (PD) was defined as progressive neurologic abnormalities or worsening neurologic status not explained by causes unrelated to tumor progression, OR a greater than 25% increase in bidimensional measurement based on MRI T2 FLAIR/T2 images, compared with the smallest measurement recorded since start of protocol therapy, OR a new lesion, OR increasing doses of corticosteroids to maintain stable neurologic status or imaging.
Study Results
Sixty-six patients, 65 eligible, enrolled on this trial (18 patients on the phase I [6 each at 50, 65, and 85 mg/m2/dose veliparib] and 48 patients on the phase II arm) from February 2012 to January 2016. One phase II patient was deemed ineligible to start therapy due to elevated alanine aminotransferase. The 6 patients treated at the MTD on the phase I component were included in the phase II analyses. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics and outcome summary
| Patient Group | All Eligible Patients n = 65 | |||||
|---|---|---|---|---|---|---|
| Eligible Phase I Patients^ n = 12^ | Eligible Phase II Patients^ n = 53^ | |||||
| n | % | n | % | n | % | |
| Age, y, at Study Enrollment | ||||||
| Median | 6.3 | – | 6.6 | – | 6.6 | – |
| Range | 2.2–15.8 | – | 2.5–12.9 | – | 2.2–15.8 | – |
| Sex | 4 | 33.3 | 33 | 62.3 | 37 | 56.9 |
| Female | ||||||
| Male | 8 | 66.7 | 20 | 37.7 | 28 | 43.1 |
| Race | 8 | 66.7 | 32 | 60.4 | 40 | 61.5 |
| White, Non-Hispanic | ||||||
| Black | 1 | 8.3 | 11 | 20.8 | 12 | 18.5 |
| Unknown | 1 | 8.3 | 7 | 13.2 | 8 | 12.3 |
| Native American | 1 | 8.3 | 1 | 1.9 | 2 | 3.1 |
| Asian | 1 | 8.3 | 1 | 1.9 | 2 | 3.1 |
| Pacific Islander | 0 | 0 | 1 | 1.9 | 1 | 1.5 |
| Ethnicity | 8 | 66.7 | 39 | 73.6 | 47 | 72.3 |
| Non-Hispanic | ||||||
| Hispanic or Latino | 3 | 25.0 | 7 | 13.2 | 10 | 15.4 |
| Unknown | 1 | 8.3 | 7 | 13.2 | 8 | 12.3 |
| Diagnosis | 10 | 83.3 | 49 | 92.5 | 59 | 90.8 |
| Brainstem glioma | ||||||
| Astrocytoma, anaplastic | 1 | 8.3 | 2 | 3.8 | 3 | 4.6 |
| Glioblastoma, NOS | 0 | 0 | 2 | 3.8 | 2 | 3.1 |
| Fibrillary astrocytoma | 1 | 8.3 | 0 | 0 | 1 | 1.5 |
| # of Maintenance Courses | 1 | 8.3 | 12 | 22.6 | 13 | 20.0 |
| 0 | ||||||
| 1 | 2 | 16.7 | 8 | 15.1 | 10 | 15.4 |
| 2 | 2 | 16.7 | 5 | 9.4 | 7 | 10.8 |
| 3 | 2 | 16.7 | 8 | 15.1 | 10 | 15.4 |
| 4 | 2 | 16.7 | 11 | 20.8 | 13 | 20.0 |
| 5 | 1 | 8.3 | 3 | 5.7 | 4 | 6.2 |
| 6 | 0 | 0 | 1 | 1.9 | 1 | 1.5 |
| 7 | 1 | 8.3 | 2 | 3.8 | 3 | 4.6 |
| 9 | 0 | 0 | 1 | 1.9 | 1 | 1.5 |
| 10 | 0 | 0 | 1 | 1.9 | 1 | 1.5 |
| 13 | 1 | 8.3 | 1 | 1.9 | 2 | 3.1 |
| # of Maintenance Courses | ||||||
| 0 | 1 | 8.3 | 12 | 22.6 | 13 | 20.0 |
| ≥1 | 11 | 91.7 | 41 | 77.4 | 52 | 80.0 |
| Median (for patients with ≥1 course) | 3 | – | 3 | – | 3 | – |
| Range (for patients with ≥1 course) | 1–13 | – | 1–13 | – | 1–13 | – |
| Best Response | 0 | 0 | 3 | 5.7 | 3 | 4.6 |
| No response assessment | ||||||
| PR | 0 | 0 | 7 | 13.2 | 7 | 10.8 |
| SD | 11 | 91.7 | 38 | 71.7 | 49 | 75.4 |
| PD | 1 | 8.3 | 5 | 9.4 | 6 | 9.2 |
^ The 6 patients treated at the MTD as part of the phase I study are included in this table as phase II patients.
Toxicity
Four patients on the phase I arm experienced DLTs during veliparib and radiation therapy. One of 6 patients treated at dose level 1 (50 mg/m2/dose) experienced a grade 2 intracranial hemorrhage, and 3 experienced DLTs at dose level 3 (85 mg/m2/dose) (2 maculopapular rash and 1 worsening of neurologic symptoms). Therefore, the RP2D of veliparib with concurrent radiation therapy was defined as 65 mg/m2 b.i.d., Monday through Friday. The most commonly observed toxicities during veliparib and radiation were lymphopenia and neutropenia; Table 2 summarizes grade 3 and higher toxicities observed.
Table 2.
Grade 3+ toxicities during radiation therapy and maintenance
| Toxicity | During Radiation (n = 64^) | During Maintenance (n = 52^) | ||
|---|---|---|---|---|
| # of Pts with Grades 3/4/5 Treatment-Related AE | % of Pts with Grades 3/4/5 Treatment-Related AE^ | # of Pts with Grades 3/4/5 Treatment-Related AE | % of Pts with Grades 3/4/5 Treatment-Related AE^ | |
| Lymphocyte count decreased | 21 | 32.8 | 26 | 50.0 |
| Neutrophil count decreased | 3 | 4.7 | 17 | 32.7 |
| White blood cell decreased | 2 | 3.1 | 16 | 30.8 |
| Platelet count decreased | 1 | 1.6 | 12 | 23.1 |
| Anemia | 1 | 1.6 | 2 | 3.8 |
| Hypokalemia | 2 | 3.1 | ||
| Rash maculopapular | 2 | 3.1 | ||
| Hydrocephalus | 1 | 1.6 | ||
| Hypertension | 1 | 1.6 | ||
| Nervous system disorders—other, specify | 1 | 1.6 | ||
| Urinary tract infection | 1 | 1.6 | ||
| Constipation | 1 | 1.6 | ||
| Hypocalcemia | 1 | 1.6 | ||
| Reversible posterior leukoencephalopathy syndrome | 1 | 1.6 | ||
| Seizure | 1 | 1.6 | ||
| Hyponatremia | 1 | 1.9 | ||
| Alanine aminotransferase increased | 1 | 1.9 | ||
| Febrile neutropenia | 1 | 1.9 | ||
| Lung infection | 1 | 1.9 | ||
| Lymphocyte count increased | 1 | 1.9 | ||
Abbreviations: Pts, patients; AE, adverse event.
^ Sixty-four eligible patients received at least some study drug and were considered evaluable for toxicity. The denominator for the RT phase was 64 and the denominator for the maintenance phase was 52 patients who started maintenance.
While receiving intrapatient dose escalation of TMZ during maintenance, 2 out of 5 patients at 175 mg/m2 and 2 of 3 patients at 200 mg/m2 experienced toxicities exceeding predefined threshold, and thus intrapatient dose escalation was halted for the remainder of the study. The most frequent grade 3 or greater toxicities during maintenance were hematologic (Table 2).
Clinical Outcomes
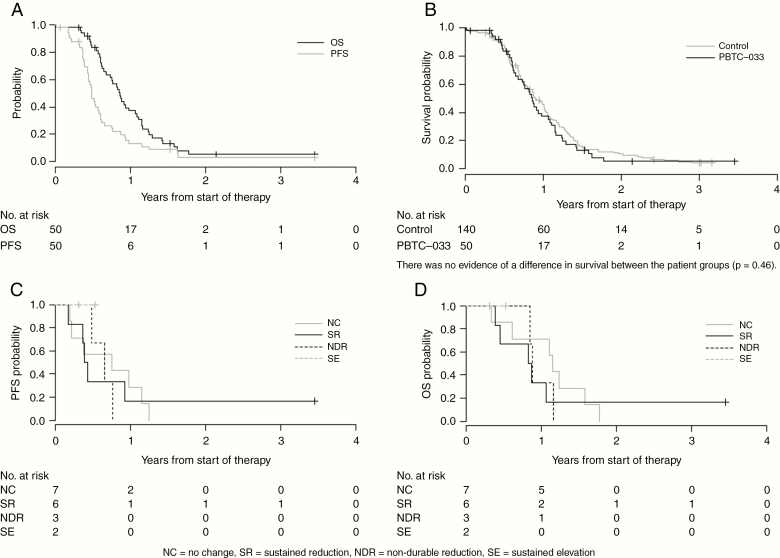
The study was stopped early following a planned futility analysis. Figure 1A shows the OS and PFS for eligible and evaluable patients included in the phase II component, and Figure 1B shows the comparison of OS to the PBTC historical control (n = 140). Fifty of 53 eligible phase II patients were evaluable for outcomes analyses. One patient withdrew prior to therapy and 2 patients received less than 1 full dose of veliparib. Median follow-up was 6.3 months (0.7–41.5 mo). One- and 2-year OS estimates were 37.2% (SE 7%) and 5.3% (SE 3%), respectively, compared with the historical control with 1-year OS of 45.6% (SE, 4.3%). Partial responses were observed in 7 of the 50 evaluable phase II patients (14%) and in 18 of the 140 historical control patients (13%).
Fig. 1.
(A) OS and PFS distributions for eligible and evaluable PBTC-033 phase II patients (n = 50). (B) OS distributions for eligible and evaluable PBTC-033 phase II patients compared with PBTC historical controls. There was no evidence of a difference in survival between the patient groups (P = 0.46). (C) PFS by PAR level changes following veliparib treatment. (D) OS by PAR level changes after veliparib treatment.
Pseudoprogression
Sixteen patients were suspected to have pseudoprogression (4 patients on the phase I arm and 12 patients on phase II). Among the 12 patients treated at the RP2D, 3 had PD at their next disease assessment and were considered to have true progression, and 7 had SD at their next assessment and therefore were classified as pseudoprogression. Two additional patients withdrew from study after initial PD assessment and had no further imaging.
Neuroimaging Results
Larger baseline tumor volumes on FLAIR were associated with worse PFS (P = 0.012) but not OS (P = 0.13). Larger enhancing tumor volumes at baseline were associated with both worse OS (P = 0.005) and PFS (P = 0.010). The baseline perfusion ratio was significantly associated with OS (P = 0.006) and PFS (P = 0.017) where hazard of death (or an event) increased as the baseline perfusion ratio increased. There was no evidence of significant association between outcome and either baseline mean ADC histogram values from FLAIR or enhancement. (Data not shown.)
Post-radiation there was significant associations in the percent change in FLAIR tumor volume from baseline with OS (P = 0.044) and PFS (P = 0.014). In addition, larger increases in tumor volume between baseline and post-radiotherapy imaging were associated with worse OS and PFS. Larger changes in enhancing tumor volume from baseline to post-radiation were associated with worse OS (P = 0.039) and PFS (P = 0.012). (Data not shown.)
Pharmacokinetics
Pharmacokinetics for veliparib (Table 3) appeared to be linear and similar to reported PK in adults.26 Mean apparent clearance (CL/F), terminal half-life (t1/2), and apparent volume of distribution (Vd/F) were similar for all dose levels. In a preceding phase I trial, patients receiving veliparib at doses of 15, 20, and 25 mg/m2 b.i.d. had mean clearances of 14.6, 10.8, and 10.9 L/m2/h, respectively.1 Those results are comparable to the clearances observed in the current study (16.1, 12.0, and 15.8 L/m2/h) at higher dose levels (50, 65, and 80 mg/m2 b.i.d.) and results from a large population PK study of adult patients (20.9 L/h, or approximately 12.1 L/m2/h adjusting for a typical body surface area of 1.73 m2).26
Table 3.
Pharmacokinetic parameters of veliparib administered concurrently with radiation
| PK Parameters | Units | Veliparib 50 mg/m2 PO b.i.d., n = 6 | Veliparib 65 mg/m2 PO b.i.d., n = 31 | Veliparib 85 mg/m2 PO b.i.d. n = 4 | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Day 1, Cmax | ng/mL | 519 | 241 | 843 | 290 | 1074 | 372 |
| Day 1, Cmax | μM | 2.12 | 0.98 | 3.45 | 1.19 | 4.40 | 1.52 |
| CL/F | L/m2/h | 16.1 | 8.1 | 12.0 | 8.1 | 15.8 | 8.3 |
| Vd/F | L/m2 | 75.4 | 29.2 | 69.8 | 98.9 | 63.9 | 10.6 |
| Terminal t1/2 | Hr | 5.18 | 6.34 | 2.27 | 2.45 | 4.45 | 4.80 |
| Day 4, Cmax | ng/mL | 409 | 84 | 734 | 284 | 954 | 348 |
| Day 4, Cmax | μM | 1.68 | 0.34 | 3.00 | 1.16 | 3.91 | 1.42 |
| Day 4, trough | ng/mL | 58 | 19 | 95 | 83 | 163 | 97 |
| Day 4, trough | μM | 0.24 | 0.08 | 0.39 | 0.34 | 0.67 | 0.40 |
PAR Levels in PBMC
PBMCs were collected from 36 patients, and 27 had pre- and post-veliparib sampling to assess treatment-induced changes in PAR. A significant change in PBMC PAR levels was defined as >50% increase or decrease from pretreatment level, at week 6 and/or week 11 after starting therapy. Two patients had inconsistent changes in post-veliparib PAR levels, with the remaining 25 patients showing consistent responses to veliparib and classified as “no change,” “sustained reduction,” with reductions in PAR levels beyond week 6, “non-durable reduction,” with reductions of PAR levels at week 3 but not at week 6, and “sustained elevation,” with increases in PAR levels beyond week 6. Seven patients had “no change,” 10 had “sustained reduction,” 5 had “non-durable reduction,” and 3 had “sustained elevation” (data not shown). PFS and OS were analyzed for these 4 subgroups and showed no association between post-veliparib PAR levels and outcome (Figure 1C, D).
Immunohistochemistry of Biopsied DIPG
Six patients had their tumors biopsied. One patient had no identifiable tumor sections on submitted slides, and 1 had tumor sections sufficient for only PARP1 testing. All 5 tumor samples showed 2+ or 3+ staining for PARP1. Components of the NHEJ pathway (KU70, KU80, DNA-PKcs) also showed 2+ to 3+ IHC staining in 4 tumors tested. Breast cancer susceptibility gene (BRCA)1 and BRCA2 from the HRR pathway also showed 3+ or 4+ IHC staining, but RAD51 staining was essentially absent in 4 tumors tested. PMS2, a component of the mismatch repair pathway, showed 3+ staining in 4 tumors tested.
ELISA Analysis of DNA Repair Proteins in PBMC
Six patients with PBMCs collected at pretreatment, during week 3 and 6 of veliparib and radiation therapy, and during maintenance therapy (week 11) were analyzed for selected DNA repair protein levels, expressed as a percentage of the pretreatment measurement (Table 4). Levels of BRCA2 and mutL homolog 1 (MLH1) showed the most pronounced effect, with 4 out of 6 patients exceeding or approaching 200% of pretreatment measurements. Moderate increases (40–60%) in KU70 levels were seen in 5 of 6 patients, and moderate decreases (40–50%) in ataxia telangiectasia mutated (ATM) were observed in 3 of 6 patients. Diverging changes in PARP1 levels were seen after veliparib treatment, with 2 patients nearly doubling their pretreatment levels at week 3 of treatment, and 2 patients showing more than 70% reductions at week 6 and/or week 11 of treatment.
Table 4.
ELISA analysis of DNA repair proteins in peripheral blood monocytes during protocol therapy
| Subject | BRCA2 | MLH1 | ATM | KU70 | PARP1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W3 (%) | W6 (%) | W11 (%) | W3 (%) | W6 (%) | W11 (%) | W3 (%) | W6 (%) | W11 (%) | W3 (%) | W6 (%) | W11 (%) | W3 (%) | W6 (%) | W11 (%) | |
| A | 100 | 85 | 80 | 89 | 106 | 222 | 99 | 97 | 106 | 126 | 142 | 146 | 189 | 131 | 186 |
| B | 145 | 503 | 309 | 93 | 103 | 169 | 116 | 125 | 138 | 144 | 125 | 101 | 202 | 240 | 260 |
| C | 84 | 99 | 105 | 116 | 132 | 237 | 98 | 118 | 86 | 148 | 108 | 103 | 112 | 92 | 85 |
| D | 170 | 208 | 136 | 59 | 67 | 213 | 62 | 59 | 65 | 101 | 147 | 132 | 27 | 35 | 73 |
| E | 148 | 72 | 189 | 85 | 94 | 89 | 64 | 56 | 50 | 108 | 118 | 133 | 21 | 80 | 29 |
| F | 193 | 197 | 154 | 90 | 102 | 99 | 85 | 63 | 47 | 153 | 168 | 120 | 86 | 102 | 93 |
W = week; each measurement reported as the mean of two readings and expressed as a percentage of the baseline value (from peripheral monocytes prior to protocol therapy). Measurements representing ≥40% increase or decrease from baseline are italicized and in bold type.
Urine Biomarkers
One hundred fifty-five urine samples were received from 53 patients, 49 of whom had pretreatment samples. Urine biomarkers from pretreatment samples were compared with 42 previously described healthy controls (healthy children undergoing MRI of the brain and spine for reasons other than a CNS tumor or vascular lesion, consented through institutional review board–approved research studies24,27,28). The controls were appropriately matched with regard to age or sex (P = 0.90 and P = 0.95, respectively).
Four putative biomarkers were identified with significant variations between cases and controls. Netrin-1 was significantly lower in the DIPG cohort at the time of diagnosis compared with controls: median (interquartile range [IQR]) 0.1 pg/µg (0.0–0.1) for cases versus 0.4 pg/µg (0.1–1.4) for controls; P < 0.001. MMP-3, TIMP-1, and bFGF were significantly higher in DIPG patients (P < 0.001 for each biomarker). The pretreatment median MMP-3 level was 1.1 pg/µg (IQR, 0.7–2.1) for cases versus 0.1 pg/µg (IQR, 0.0.–0.3) for controls. Median TIMP-1 and bFGF levels were 7.3 pg/µg (IQR, 5.3–10.9) and 3.3 pg/µg (IQR, 2.1–5.6) for cases compared with 4.0 pg/µg (IQR, 2.5–6.6) and 0.8 pg/µg (IQR, 0.2–3.4) for controls (data not shown). In logistic regression models adjusted for sex and age, each biomarker was associated with DIPG compared with controls.
Based on cut-point analyses, measurement of urinary levels of these 4 molecules exhibited accuracy up to 79% and sensitivity up to 100% for identifying the presence of DIPG in a child (Table 5). Of these, TIMP-1 in our patient cohort was observed to be a significant predictor of PFS, with increasing values at the time of baseline conferring improved outcome hazard ratio = 0.92 (95% CI: 0.85–1.00), P = .048; data not shown.
Table 5.
Summary of diagnostic performance of urinary biomarkers in differentiating DIPG patients and controls
| Biomarker | Area Under the Curve (95% CI) | Cut-point Based on Youden’s Index | Sensitivity Based on Cut-point | Specificity Based on Cut-point | Accuracy |
|---|---|---|---|---|---|
| Netrin-1 | 0.77 (0.66–0.87) | 0.536 | 98% | 49% | 76% |
| bFGF | 0.75 (0.64–0.86) | 0.419 | 96% | 38% | 70% |
| TIMP-1 | 0.74 (0.63–0.84) | 0.541 | 100% | 8% | 58% |
| MMP-3 | 0.85 (0.76–0.93) | 0.529 | 78% | 81% | 79% |
Discussion
Based on preclinical studies showing enhancement of TMZ and radiation efficacy by veliparib,19,29–31 we initiated this phase I/II trial of veliparib and radiation therapy followed by veliparib and TMZ in children with newly diagnosed DIPGs. While the novel combination therapy was generally tolerable, this strategy failed to improve survival compared with a contemporary PBTC historical series.
Our patients’ response to PARP inhibition, as measured by PAR formation, appeared to be heterogeneous. Of 25 patients with PBMC samples from pretreatment through week 11 of protocol therapy, 15 had >50% PAR reduction, with 10 (40%) patients showing persistent inhibition through week 11. In a study of 40 healthy adults, a similar percentage (47.5%) showed >50% PAR reduction in peripheral PBMC after ex vivo veliparib treatment, while several individuals showed resistance to veliparib.32 For the patients on this trial, the degree of PAR reduction in PBMCs did not correlate with survival; whether PAR reduction in PBMCs or other surrogate markers of PARP inhibition reflect intratumoral effect and/or correlate with clinical outcomes remains to be explored.
Similar to results from prior PBTC trials for DIPG, baseline intratumoral enhancement was associated with shorter survival,33,34 which is likely a marker of more aggressive biologic behavior.35 Similarly, higher baseline intratumoral perfusion values were associated with shorter survival, which is consistent with association between increased vascularity and higher-grade tumors.36,37 Changes in tumor volume post-radiation were also associated with survival.
A secondary objective was to investigate the utility of urinary biomarkers in identifying disease presence, monitoring progression, and predicting outcome. The use of urinary biomarkers in pediatric brain tumors has been previously reported, but not for patients with DIPG.24,25,27,28 In this trial, several markers successfully distinguished the presence of DIPG in patients versus healthy controls, even when evaluated as single-species markers (MMP-3, TIMP-1, bFGF, and netrin-1) with an accuracy up to 79% and sensitivity up to 100%. Based on previous reports, we anticipate that combining 2 or more markers (multiplexing) may markedly improve the robustness of this approach.24 One biomarker demonstrated significance in predicting survival (MMP-9) and progression-free survival (TIMP-1), while another (MMP-13) correlated with baseline tumor volume on imaging. These preliminary findings serve as successful proof-of-principle data supporting the continued investigation of urinary biomarkers as a novel, non-invasive diagnostic and prognostic technique for children with DIPG.
While several PARP inhibitors are now FDA approved for BRCA-deficient breast and ovarian cancers, identification of consistent biomarkers predicting response and elucidation of mechanisms of resistance to these drugs have lagged. Initial studies showed that HRR and NHEJ repair proteins such as BRCA1/2 and RAD51 are elevated after PARP inhibition and likely mediate resistance, while deficiencies in ATM or RAD51 predict hypersensitivity to PARP inhibitors. In our trial, in the limited number of patients whose PBMCs were analyzed for DNA repair protein levels after veliparib treatment, we observed significant elevation of BRCA2 and modest reduction in ATM, suggesting that these biomarkers may predict responses to PARP inhibitor treatment in future trials.
More recent studies have shown complex, multifaceted mechanisms of resistance to PARP inhibition in various cancers, including mutation,38 methylation,39 and myriad of other molecular mechanisms, delineated in a recent review article.40 These data suggest that clinical responses to PARP inhibition are likely heterogeneous, and resistance is likely inevitable in a high percentage of patients; however, these data can potentially guide rational design of future combination therapies to overcome treatment resistance. For example, GBMs with unmethylated O6-methylguanine-DNA methyltransferase promoters are predicted to be sensitive to veliparib and radiation therapy30,41 but resistant to veliparib and TMZ.41 This suggests that following completion of veliparib and radiation treatment, a combination of veliparib with a histone deacetylase inhibitor42 or a cyclin-dependent kinase inhibitor43 is likely to be more effective than veliparib plus TMZ. Ultimately, in future trials, prediction of tumor sensitivity to PARP inhibition and selection of the optimal combination with other targeted therapies will be improved through routine molecular characterization of newly diagnosed tumors through whole-genome analysis44 and other “omic” technologies. Although combining veliparib, radiation, and TMZ failed to improve survival in children with DIPG, drug entry and biological efficacy may differ between brainstem and non-brainstem high-grade gliomas, and an ongoing Children’s Oncology Group trial is evaluating such a strategy combining veliparib, radiation, and TMZ for pediatric high-grade gliomas without H3K27M mutations. An adult phase I/II trial of veliparib, radiation, and TMZ in newly diagnosed glioblastoma (NCT00770471) completed accrual in 2017, the results of which should lead to more insights regarding the utility of PARP inhibition in CNS tumors. Based on the preliminary data from this trial, PBTC will continue to explore the use of urinary biomarkers as a novel diagnostic and prognostic tool in future DIPG trials.
Funding
The trial was funded by a National Cancer Institute CTEP PBTC U01 grant (2UM1CA081457 [UM1]) and the American Lebanese Syrian Associated Charities. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflict of interest statement
P.A. is an employee of AbbVie Pharmaceutical and shareholder. V.G. was an employee of AbbVie Pharmaceutical until 2017 and is a shareholder. I.D. declares advisory roles for Roche and Apexigen and consultant roles for Celgene, BMS, and Pfizer. A.O-T. reports advisory roles for Roche and Lilly. L.K. reports advisory role for Roche. All others report no conflicts.
Authorship statement
Experimental design: P.B., J.S., A.O-T., C.B, X., T.Y.P., E.S., P.T., A.A., P.A.,V.G., A.P., L.K., S.B. Implementation: P.B., J.S., A.O-T., C.B., T.Y.P, E.S., P.T., A.A., P.A.,V.G., A.P., M.F. Acquisition and analysis/interpretation of data: P.B., J.S., A.O-T., C.B, X.N-L., T.Y.P., E.S., P.T., A.A., P.A.,V.G., L.K., I.Q., A.B., I.D., M.F. Preparation/review of manuscript: all authors.
References
- 1. Su JM, Thompson P, Adesina A, et al. . A phase I trial of veliparib (ABT-888) and temozolomide in children with recurrent CNS tumors: a pediatric brain tumor consortium report. Neuro Oncol. 2014;16(12):1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38(1):27–35. [DOI] [PubMed] [Google Scholar]
- 3. Cohen KJ, Heideman RL, Zhou T, et al. . Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta N, Goumnerova LC, Manley P, et al. . Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol. 2018;20(11):1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu G, Broniscer A, McEachron TA, et al. ; St Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castel D, Philippe C, Calmon R, et al. . Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackay A, Burford A, Carvalho D, et al. . Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith SJ, Long A, Barrow JH, Macarthur DC, Coyle B, Grundy RG. Pediatric high-grade glioma: identification of poly(ADP-ribose) polymerase as a potential therapeutic target. Neuro Oncol. 2011;13(11):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zarghooni M, Bartels U, Lee E, et al. . Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28(8):1337–1344. [DOI] [PubMed] [Google Scholar]
- 10. Chornenkyy Y, Agnihotri S, Yu M, et al. . Poly-ADP-ribose polymerase as a therapeutic target in pediatric diffuse intrinsic pontine glioma and pediatric high-grade astrocytoma. Mol Cancer Ther. 2015;14(11):2560–2568. [DOI] [PubMed] [Google Scholar]
- 11. Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108(8):3406–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Yu X. The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene. 2015;34(26):3349–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chalmers AJ, Lakshman M, Chan N, Bristow RG. Poly(ADP-ribose) polymerase inhibition as a model for synthetic lethality in developing radiation oncology targets. Semin Radiat Oncol. 2010;20(4):274–281. [DOI] [PubMed] [Google Scholar]
- 16. Donawho CK, Luo Y, Luo Y, et al. . ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13(9):2728–2737. [DOI] [PubMed] [Google Scholar]
- 17. Han HS, Diéras V, Robson M, et al. . Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29(1):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Albert JM, Cao C, Kim KW, et al. . Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13(10):3033–3042. [DOI] [PubMed] [Google Scholar]
- 19. Lemasson B, Wang H, Galbán S, et al. . Evaluation of concurrent radiation, temozolomide and ABT-888 treatment followed by maintenance therapy with temozolomide and ABT-888 in a genetically engineered glioblastoma mouse model. Neoplasia. 2016;18(2):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muscal JA, Thompson PA, Giranda VL, et al. . Plasma and cerebrospinal fluid pharmacokinetics of ABT-888 after oral administration in non-human primates. Cancer Chemother Pharmacol. 2010;65(3):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta MP, Wang D, Wang F, et al. . Veliparib in combination with whole brain radiation therapy in patients with brain metastases: results of a phase I study. J Neurooncol. 2015;122(2):409–417. [DOI] [PubMed] [Google Scholar]
- 22. Thall PF, Wooten LH, Tannir NM. Monitoring event times in early phase clinical trials: some practical issues. Clin Trials. 2005;2(6):467–478. [DOI] [PubMed] [Google Scholar]
- 23. Liu X, Palma J, Kinders R, et al. . An enzyme-linked immunosorbent poly(ADP-ribose) polymerase biomarker assay for clinical trials of PARP inhibitors. Anal Biochem. 2008;381(2):240–247. [DOI] [PubMed] [Google Scholar]
- 24. Pricola Fehnel K, Duggins-Warf M, Zurakowski D, et al. . Using urinary bFGF and TIMP3 levels to predict the presence of juvenile pilocytic astrocytoma and establish a distinct biomarker signature. J Neurosurg Pediatr. 2016;18(4):396–407. [DOI] [PubMed] [Google Scholar]
- 25. Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14(8):2378–2386. [DOI] [PubMed] [Google Scholar]
- 26. Salem AH, Giranda VL, Mostafa NM. Population pharmacokinetic modeling of veliparib (ABT-888) in patients with non-hematologic malignancies. Clin Pharmacokinet. 2014;53(5):479–488. [DOI] [PubMed] [Google Scholar]
- 27. Smith ER, Manfredi M, Scott RM, Black PM, Moses MA. A recurrent craniopharyngioma illustrates the potential usefulness of urinary matrix metalloproteinases as noninvasive biomarkers: case report. Neurosurgery. 2007;60(6):E1148–1149; discussion E1149. [DOI] [PubMed] [Google Scholar]
- 28. Akino T, Han X, Nakayama H, et al. . Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma. Cancer Res. 2014;74(14):3716–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Vuurden DG, Hulleman E, Meijer OL, et al. . PARP inhibition sensitizes childhood high grade glioma, medulloblastoma and ependymoma to radiation. Oncotarget. 2011;2(12):984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jue TR, Nozue K, Lester AJ, et al. . Veliparib in combination with radiotherapy for the treatment of MGMT unmethylated glioblastoma. J Transl Med. 2017;15(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta SK, Kizilbash SH, Carlson BL, et al. . Delineation of MGMT hypermethylation as a biomarker for veliparib-mediated temozolomide-sensitizing therapy of glioblastoma. J Natl Cancer Inst. 2016;108(5) pii: djv369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ji J, Kinders RJ, Zhang Y, et al. . Modeling pharmacodynamic response to the poly(ADP-ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells. PLoS One. 2011;6(10):e26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poussaint TY, Kocak M, Vajapeyam S, et al. . MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro Oncol. 2011;13(4):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poussaint TY, Vajapeyam S, Ricci KI, et al. . Apparent diffusion coefficient histogram metrics correlate with survival in diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2016;18(5):725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Conway AE, Reddick WE, Li Y, et al. . “Occult” post-contrast signal enhancement in pediatric diffuse intrinsic pontine glioma is the MRI marker of angiogenesis? Neuroradiology. 2014;56(5):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vajapeyam S, Stamoulis C, Ricci K, Kieran M, Poussaint TY. Automated processing of dynamic contrast-enhanced MRI: correlation of advanced pharmacokinetic metrics with tumor grade in pediatric brain tumors. AJNR Am J Neuroradiol. 2017;38(1):170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hipp SJ, Steffen-Smith E, Hammoud D, Shih JH, Bent R, Warren KE. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol. 2011;13(8):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pettitt SJ, Krastev DB, Brandsma I, et al. . Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat Commun. 2018;9(1):1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kondrashova O, Topp M, Nesic K, et al. ; Australian Ovarian Cancer Study (AOCS) Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9(1):3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim Y, Kim A, Sharip A, et al. . Reverse the resistance to PARP inhibitors. Int J Biol Sci. 2017;13(2):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barazzuol L, Jena R, Burnet NG, et al. . Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiat Oncol. 2013;8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rasmussen RD, Gajjar MK, Jensen KE, Hamerlik P. Enhanced efficacy of combined HDAC and PARP targeting in glioblastoma. Mol Oncol. 2016;10(5):751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson N, Li YC, Walton ZE, et al. . Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2011;17(7):875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jenner ZB, Sood AK, Coleman RL. Evaluation of rucaparib and companion diagnostics in the PARP inhibitor landscape for recurrent ovarian cancer therapy. Future Oncol. 2016;12(12):1439–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]