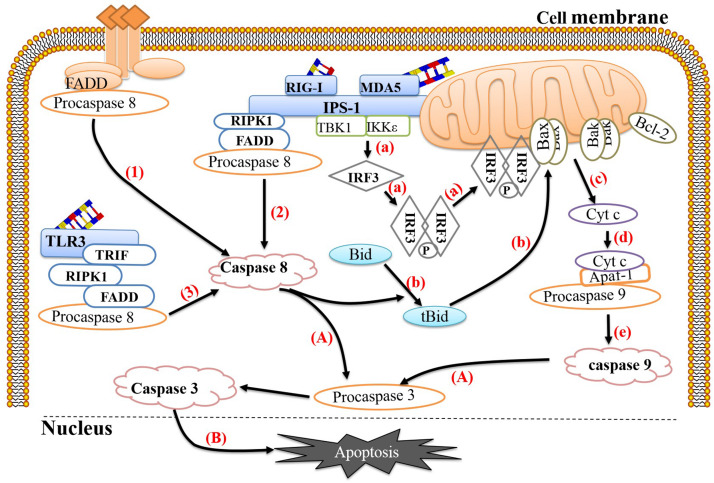
FIGURE 1.
Link between pathogen recognition and apoptosis. (1) The death ligands TNF and FasL bind to their corresponding receptors, TNFR and Fas, and recruit TRADD and FADD, respectively, which then bind to procaspase 8 to form the death-inducing signaling complex (DISC) to activate caspase 8. (2) RIG-1 or MDA-5 recognizes viral dsRNA and recruits IPS-1. Activated IPS-1 recruits FADD and RIPK1 to form a complex and then recruits and activates procaspase 8. (3) TLR-3 recognizes viral dsRNA in the cytoplasm and exposes the TIR domain. The adaptor TRIF containing the TIR domain binds to RIPK. The C-terminus of RIPK1 has a death domain (DD), which can interact with FADD, after which FADD recruits and activates procaspase 8. (a) Activated IPS-1 can also recruit IKKε and TBK1, causing phosphorylation and dimerization of IRF-3. The IRF-3 homodimer can translocate to the mitochondrial membrane and form a complex with Bax. (b) Activated caspase 8 can cleave Bid to form tBid with proapoptotic activity, which can induce Bax and Bak to insert into the outer mitochondrial membrane. (c) Bax or Bak undergoes conformational changes and forms heterodimers or homodimers, thereby destroying the mitochondrial membrane and reducing the mitochondrial membrane potential. Subsequently, mitochondria release Cyt c into the cytoplasm. (d,e) Cyt c, procaspase 9 and Apaf-1 combine to form a complex that cleaves procaspase 9 into caspase 9. (A) Caspase 9 and caspase 8 cleave procaspase 3 into caspase 3. (B) Caspase 3 translocates to the nucleus and activates proteins, ultimately leading to apoptosis. The image content has been borrowed from other articles in the literature (Croft et al., 2017; Sun et al., 2019).