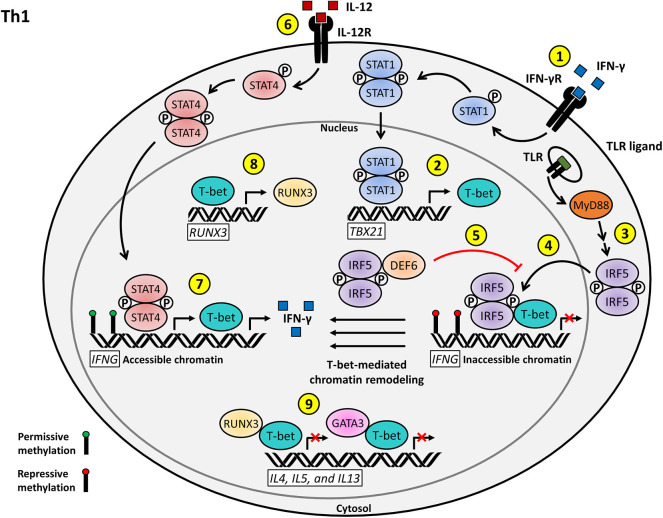
Figure 1.
Proposed model for the T cell-intrinsic role of IRF5 as a positive regulator of Th1 effector function and differentiation. (1) IFN-γ stimulation of the IFN-γR on naïve CD4+ T cells induces STAT1 activation and nuclear translocation. (2) Phosphorylated STAT1 activates the transcription of TBX21, leading to the production of T-bet. (3) T cell stimulation, possibly through TLR signaling, induces IRF5 activation and nuclear translocation. (4) Nuclear IRF5 recruits T-bet to the silenced IFNG locus to facilitate permissive T-bet-mediated chromatin remodeling. (5) DEF6 binds to nuclear IRF5 in order to inhibit IRF5-mediated T-bet recruitment to the IFNG locus. (6) IL-12 signaling through the IL-12R results in STAT4 activation and nuclear translocation. (7) Phosphorylated STAT4 and T-bet induce the transcription of the accessible IFNG locus and subsequent IFN-γ signaling drives Th1 effector differentiation. (8) T-bet also acts as a positive regulator of RUNX3 transcription. (9) T-bet interacts with RUNX3 and GATA3 to inhibit the transcription of Th2 signature genes, including IL4, IL5, and IL13, to promote Th1 polarization.