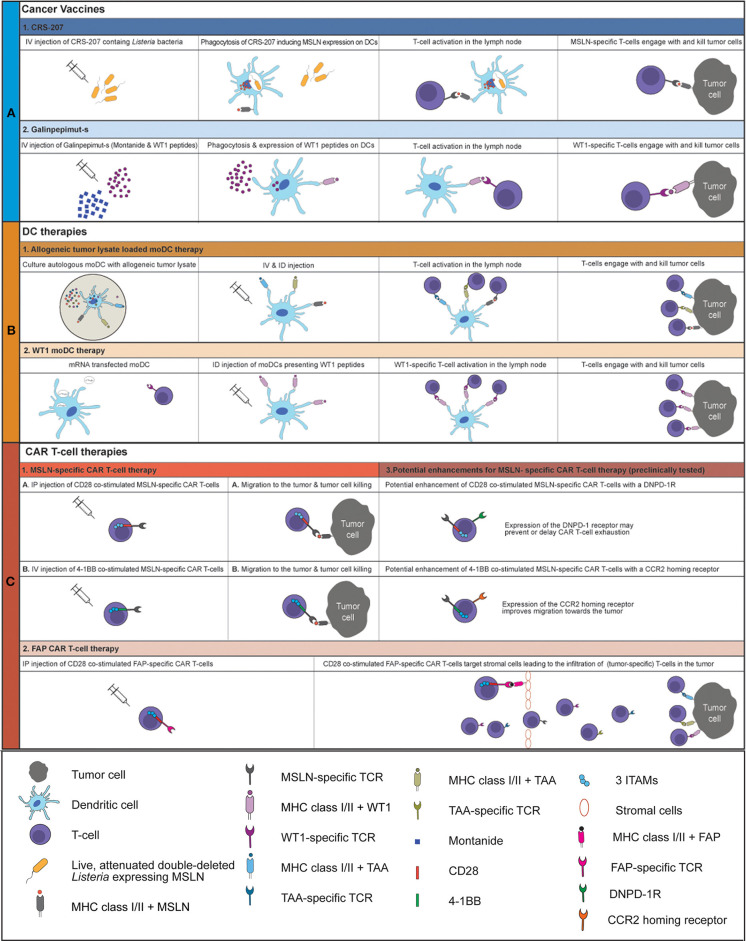
Figure 1.
Overview of current clinically tested cancer vaccines and cellular therapies for MPM An overview of the working mechanism of CRS-207 (A1), Galinpepimut-s (A2), allogeneic tumor lysate loaded moDC therapy (B1), WT1 moDC therapy (B2), MSLN-specific CD28 co-stimulated CAR T-cell therapy (C1A), MSLN-specific 4-1BB co-stimulated CAR T-cell therapy (C1B) and FAP CAR T-cell therapy (C2). The potential enhancements of MSLN-specific CAR T-cell therapy are displayed in C3. IV, intravenous; ID, intradermal; IP, intrapleural; MSLN, mesothelin; moDC, monocyte-derived dendritic cell; MHC, major histocompatibility complex; TCR, T-cell receptor; WT1, Wilms Tumor 1 protein; TAA, tumor-associated antigen; ITAM, Immunoreceptor tyrosine-based activation motif; DNPD-1R, dominant negative PD1 receptor; CCR2, CC chemokine receptor 2; FAP, fibroblast activation protein.