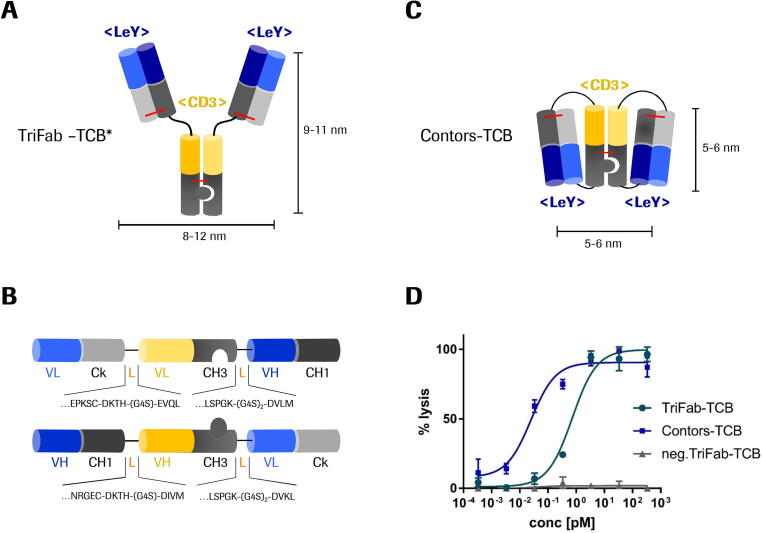
Fig. 4.
A) TriFab format with two Fab moieties recognizing the Lewis Y antigen (blue) and one Fv directed against the CD3ε antigen (yellow) according to Dickopf et al. (*)[16]. Interdomain disulfide bridges are indicated in red. B) The equivalent binders described in the TriFab format were used to design a two-chained format that is related to the Contorsbody technology by Georges et al. 2020 [64]. Molecules were produced in HEK suspension culture expression systems by transient transfection. C) After kappa-select affinity capture, 85% of the protein presented as folded TriFab-Contorsbody. As side product a tandem-like Fab molecule (9%) and high-molecular weight species (aggregates) were also observed which could be removed by SEC. Preferential assembly of the Contors-TriFab format from these input molecules occurs because during translation and folding, intrachain assembly/and disulfides appear to form earlier than the interchain assembly of knob-into-hole CH3 and VH/VL. The final yields of purified TriFab-Contorsbodies was 8 mg per liter culture. D) Lewis Y targeting TriFab-TCB and Contorsbody-TCB were applied to co-culturing assays of LeY-positive MCF-7 cells and human PBMC (E:T ratio = 10:1). Results are expressed as mean and SD from triplicate wells and plotted as 3-parameter non-linear regression fitting using Graphpad Prism software. Representative plot of three independent experiments is shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)