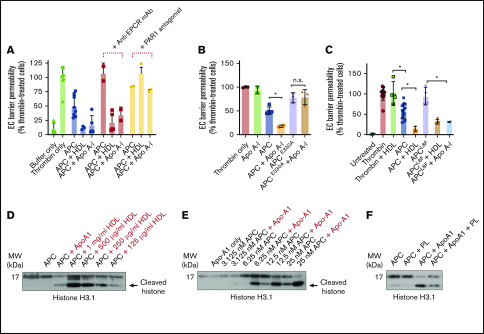
Figure 2.
Apo A-I enhances endothelial cell (EC) barrier integrity and extracellular histone proteolysis by APC. (A) HDL- and Apo A-I–enhanced protection against thrombin-induced disruption of the EC barrier by APC (10 nM; blue) was measured in the presence of an anti-EPCR antibody (25 µg/mL, RCR-252) that blocks APC–EPCR binding (red) or with a PAR1 antagonist (SCH5300348; yellow) that prevents APC-dependent PAR1 signaling. (B) To confirm the role of PAR1 in Apo A-I–enhanced APC EC barrier protection, Apo A-I–dependent enhancement of either wild-type APC, or an APC variant that is unable to recognize PAR1 (APCE330A, both 10 nM), was characterized. (C) Similarly, the role of APC–EPCR binding in Apo A-I–stimulated APC signaling function was assessed by comparing protection of endothelial barrier integrity by wild-type APC and an APC variant unable to bind EPCR (APCL8F, both 10 nM). (D) Proteolysis of histone H3.1 by APC (6.25 nM) was assessed with HDL (125 µg/mL to 1 mg/mL) or Apo A-I (150 µg/mL) by western blotting with an antihistone H3.1 antibody, which recognizes both APC-cleaved and APC-uncleaved fragments of histone H3.1. (E) Similarly, proteolysis of histone H3.1 by titration of APC (3.25-25 nM) was assessed ± Apo A-I (150 µg/mL). (F) In addition, the ability of anionic phospholipids (20 µM) to modulate Apo A-I–mediated enhanced histone proteolysis by APC (6.25 nM) was assessed by using the same approach. Experiments were performed in at least triplicate, and results are presented as the mean ± standard deviation. Statistical significance was determined by using the Student t test. *P < .05. mAb, monoclonal antibody.