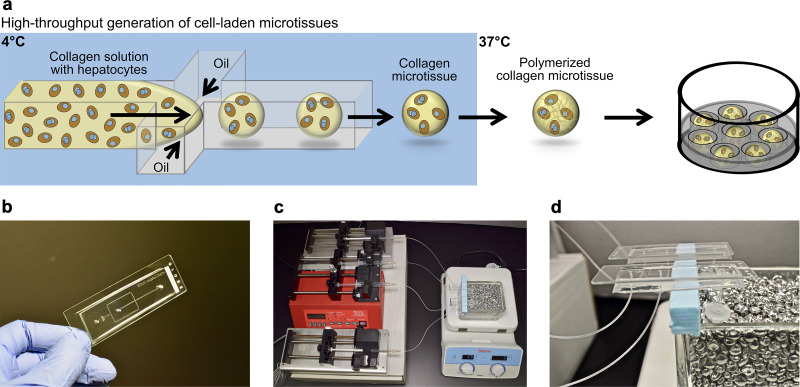
Figure 1.
Fabrication of primary human hepatocyte (PHH) microtissues via droplet microfluidics. (a) Hepatocytes are suspended in pH-neutralized collagen solution and then perfused through a droplet generating microfluidic device (see Materials and Methods for details about device dimensions). Oil is perfused at a rate ∼4 times faster than the aqueous phase to produce microtissues. Microtissues are formed using the microfluidic device at 4°C and collected at 37°C to promote the rapid polymerization of the collagen droplets and encapsulation of the cells within the droplets. Oil is removed and polymerized microtissues are resuspended in culture medium and subsequently seeded into agarose (2% w/v) microwells cast within multiwell plates. The hepatocytes can be cocultured with nonparenchymal cell (NPC) types by either coencapsulating both cell types within the microtissue or by seeding/coating the NPCs onto the surface of the polymerized collagen-based hepatic microtissues. (b) Polydimethylsiloxane (PDMS)-based microfluidic devices consisting of a single emulsion droplet generator with 300-μm straight channel and 150-μm nozzle used to fabricate microtissues. (c) Microtissue fabrication setup to create microtissues. Two parallel microfluidic devices with syringes containing either oil or collagen + cells are shown; however, additional devices can be run simultaneously to increase fabrication throughput. (d) Microfluidic devices with collection tubes. The microtissues are collected into a 2-ml collection tube connected to the microfluidic device with a short outlet tubing. The collection tube is kept at 37°C in a heated bead bath to ensure immediate polymerization of the microtissues as they are dispensed into the tube.