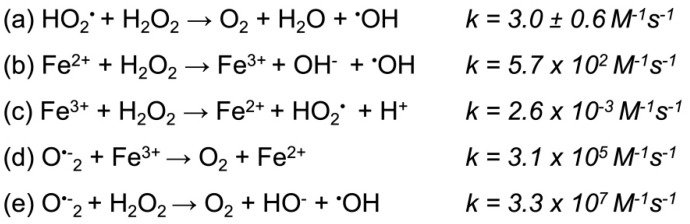Figure 5.
Haber–Weiss reaction scheme. The Haber–Weiss reaction generates hydroxyl radicals from hydrogen peroxide, and superoxide catalyzed by iron ions; k, reaction rate coefficient. (a) The reaction is too slow and cannot affect cellular processes. (b) A catalyst (iron) was used, which was already known in the Fenton reaction. (c) Iron ions formed in the Fenton reaction were reduced to iron ions by hydrogen peroxide. (d) In order for the reaction to proceed at a faster rate, a reducing agent was added: superoxide anion radical. (e) As a result of the b and d reactions and iron ions, the Haber–Weiss reaction was obtained, which proceeds at a high rate according to [78,79,80].