Figure 3. Notch1+/− aortic valve interstitial cells promote calcification and macrophage maturation in vitro through altered cytokine secretion.
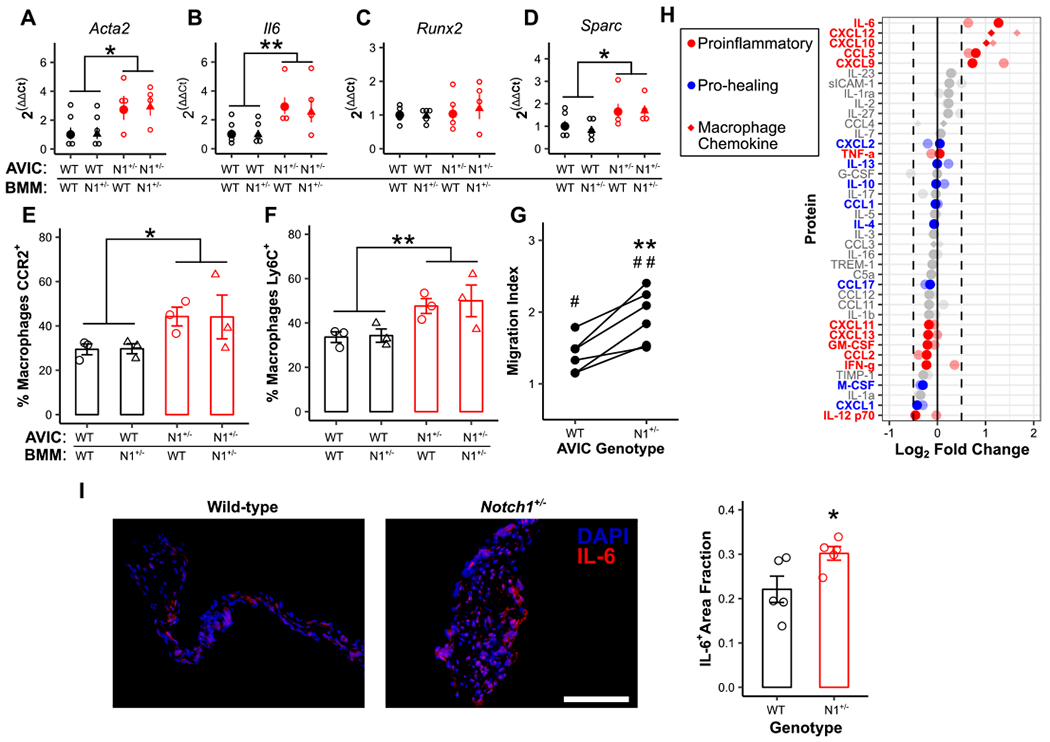
Wild-type (WT) and Notch1+/− (N1+/−) aortic valve interstitial cells (AVICs) were cultured with WT and N1+/− bone marrow-derived macrophages (BMMs) and assayed for transcription of common markers of dystrophic (A, B) and osteogenic (C, D) calcification (N = 4). Flow cytometry for CCR2 (E) and Ly6C (F) expression was performed on BMMs (black = WT AVICs; red = N1+/− BMMs; circle = WT BMMs, triangle = N1+/− BMMs, N = 3). (G, N = 6) Migration of WT macrophages in the presence of either WT or N1+/− AVIC-cultured media normalized to uncultured media controls. (H) Cytokine microarray analysis of secreted media from WT and N1+/− AVICs. Data is plotted as fold change in N1+/− AVIC-cultured media compared to WT control. Full color points are cultured media; faded points are cell lysates. Points represent an average of two experiments with 3 pooled samples each. (I, N = 5) IL-6 (red) expression in WT and N1+/− aortic valves; scale bar = 200 μm. All summary data represent mean ± s.e.m. Data were analyzed by two-way ANOVA on untransformed ΔCt values (A-D) or flow populations (E, F), or one-way ANOVA followed by paired, two-tailed t tests with Holm-Sidak corrections (G), or two-tailed t test (I). *P < 0.05 from wild-type AVICs, **P < 0.01 from wild-type AVICs, #P < 0.05 from monoculture control, ##P < 0.01 from monoculture control. N = biological replicates.
