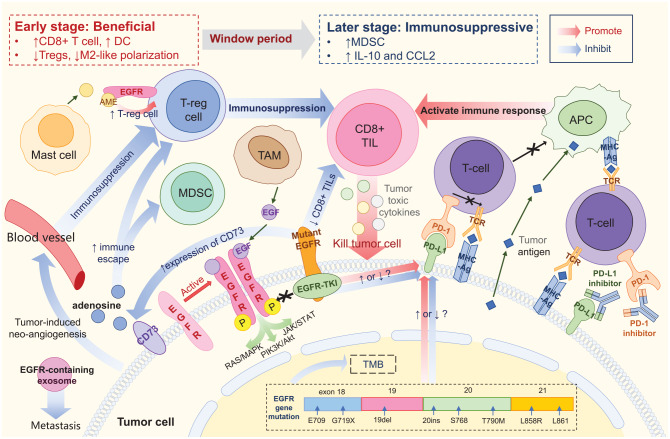
Figure 1.
Tumor microenvironment of EGFR-mutant NSCLC. EGFR protein expressed on the tumor cell surface could be activated by homodimerization, and may then activate downstream pathways. Mutant EGFR protein could bind to EGFR-TKI, thus blocking signal transduction. PD-L1 binding to PD-1 prevents T cells from recognizing tumor cells, thereby causing immune escape. PD-(L)1 inhibitor blocks PD-1 or PD-L1, promoting the binding of TCR on T cells to MHC molecules on tumor cells and reinitiating the immune response. Tumor antigens can be directly taken up by APC and presented to T cells. CD8+ TIL are major effector cells that produce an immune-mediated tumor-killing effect by secreting tumor toxic cytokines. EGFR mutation is associated with reduced density and function of CD8+ TIL, resulting in a poor response to ICIs. Treg is the predominant immunosuppressive cell type. AME released by mast cells could bind to EGFR expressed on Treg, promoting the function of Treg and indirectly promoting immunosuppression. CD73 expressed on tumor cells may induce the formation of adenosine, further promoting Treg and MDSC and hindering the immune response. Mutant EGFR is associated with upregulation of CD73. EGFR-containing exosomes are secreted by tumor cells and may drive metastasis. Tumor-induced neoangiogenesis leads to immunosuppression by promoting immunosuppressive cells and inhibiting effector T cells. Meanwhile, EGFR mutation is associated with reduced TMB, but its effect on PD-L1 expression is still controversial. Application of EGFR-TKI is suspected to cause dynamic changes in PD-L1 expression, but the specific effects remain unclear. The TME of EGFR-aberrant NSCLC represents a dynamic transition from beneficial in the early stages to immunosuppressive in later stages during EGFR-TKI treatment. Ag, antigen; AME, amphiregulin; APC, antigen presenting cell; CCL2, C–C motif chemokine ligand 2; DC, dendritic cell; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; IL-10, interleukin-10; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; NSCLC, non-small-cell lung cancer; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor; TIL, tumor-infiltrating lymphocyte; TKI, tyrosine kinase inhibitor; TAM, tumor-associated macrophage; TMB, tumor mutation burden; TME, tumor microenvironment; Treg, regulatory T cell