Abstract
Introduction
We tested the impact of antiepileptic drug (AED) administration on post-cardiac arrest epileptiform electroencephalographic (EEG) activity.
Methods
We studied an observational cohort of comatose subjects treated at a single academic medical center after cardiac arrest from September 2010 to January 2018. We aggregated the observed EEG patterns into 5 categories: suppressed; discontinuous background with superimposed epileptiform activity; discontinuous background without epileptiform features; continuous background with epileptiform activity; and continuous background without epileptiform activity. We calculated overall probabilities of transitions between EEG states in a multistate model, then used Aalen’s additive regression to test if AEDs or hypothermia are associated with a change in these probabilities.
Results
Overall, 828 subjects had EEG-monitoring for 42,840 hours with a median of 40 [IQR 23 to 64] hours per subject. Among patients with epileptiform findings on initial monitoring, 50% transitioned at least once to a non-epileptiform, non-suppressed state. By contrast, 19% with non-epileptiform initial activity transitioned to an epileptiform state at least once. Overall, 568 (78%) patients received at least one AED. Among patients with continuous EEG background activity, valproate, levetiracetam and lower body temperature were each associated with an increased probability of transition from epileptiform states to non-epileptiform states, where patients with discontinuous EEG background activity no agent linked to an increased probability of transitioning from epileptiform states.
Conclusion
After cardiac arrest, the impact of AEDs may depend on the presence of continuous cortical background activity. These data serve to inform experimental work to better define the opportunities to improve neurologic care post-cardiac arrest.
Keywords: cardiac arrest, anoxic brain injury, seizure, antiepileptic drug, ictal, electroencephalography
Introduction
Sudden cardiac arrest affects over 500,000 people in North America each year.1 With advances in care, rates of return of spontaneous circulation (ROSC) and survival to intensive care unit admission are increasing,2,3 but nearly half of initial survivors die from sequelae of anoxic brain injury.4 Electroencephalographic (EEG) patterns on the ictal-interictal spectrum develop in approximately 1 in 3 comatose post-arrest patients and are associated with worse outcomes.5–7 Because of this prevalence, the American Heart Association recommends EEG monitoring frequently or continuously in comatose post-arrest patients.8 However, it is unknown whether or not epileptiform EEG patterns after cardiac arrest are a treatable cause of secondary brain injury or simply a sign of completed primary brain damage.
EEG changes dynamically during the hours to days after cardiac arrest.9–12 Some findings, like discontinuous background activity, may normalize spontaneously as the energetics and function of cortical neurons improve.9,11 Resolution is associated with favorable recovery, but may be affected by medications, hypothermia, or other physiological variables.9,11 Other patterns, like some subtypes of post-anoxic myoclonus or burst suppression with identical bursts, occur in the setting of diffuse cortical and subcortical damage, evolve in a stereotypical manner and ultimately dissipate without evidence that AED therapy affects this progression.12–14 We quantified the probabilities of transition between various abnormal and normal EEG patterns in comatose post-arrest patients using a multi-state probability model. To better understand the potential role of AED therapy, we leveraged clinical variation in the timing of AED administration. Specifically, we tested if AED administration was associated with an increase in the probability that epileptiform EEG patterns resolve compared to the probability of spontaneous resolution.
Methods
Patients and setting
We performed an observational cohort study of patients treated at a single academic medical center after resuscitation from cardiac arrest from September 2010 to January 2018. The University of Pittsburgh Institutional Review Board the study design with a waiver of informed consent. We excluded patients who did not undergo EEG monitoring because of rapid awakening and those with early limitations in care or death because of pre-existing advanced directives, refractory multi-system organ failure, rearrest, or lack of available EEG recording devices. We further excluded patients with no available medication data returned from an automated query of the electronic medical record and those with arrest due to trauma or a primary neurological event.
We maintain a prospective registry of all patients treated by our Post-Cardiac Arrest Service that includes demographic and disease-specific baseline characteristics, treatments and outcomes. Our practice is and was to monitor all comatose survivors of cardiac arrest with continuous EEG. We also administer AEDs in a standardized fashion to treat potentially epileptiform EEG activity as previously described.9 We actively managed temperature in comatose patients to target 33°C or 36°C for 24 hours regardless of initial rhythm or arrest location. Thereafter, we rewarmed patients at 0.25°C/hr to normothermia, which we maintained until 72 hours post-arrest. During the study period, sedation typically used a combination of propofol and fentanyl. After 2014, we avoided use of benzodiazepines for sedation unless propofol resulted in hemodynamic instability or benzodiazepines were used as an anticonvulsant.
EEG and AED data
Our hospital has around-the-clock in-house technologists who initiate EEG monitoring on ICU arrival, an average of 6–8h after return of spontaneous circulation (ROSC).9 We used 22 gold-plated cup electrodes placed in the standard 10–20 International System of Electrode Placement and record data using XLTech Natus® Neuroworks digital video/EEG systems (Natus Medical Inc., Pleasanton, CA). We typically continued EEG monitoring until awakening, death, or until approximately 48h of data without any findings considered actionable by the treating team.
Two study coauthors (PJC and MAB, a board certified epileptologist) jointly reviewed and annotated all EEG recordings; these two discussed discordant interpretations until achieving agreement. Informed by previous research on prognostication after cardiac arrest and standard terminology,9–12,15 we separately recorded each change in the EEG. Consistent with American Clinical Neurophysiology Society (ACNS) guidelines, we categorized background activity as: suppressed (<10uV); suppression-burst (“burst-suppression” or “discontinuous”); continuous with periods of attenuation (i.e. “nearly continuous”); or continuous. We categorized superimposed patterns as: generalized suppression; seizures; polyspike-burst with myoclonus; polyspike-wave discharges without myoclonus; generalized periodic discharges ≤2.5Hz; generalized periodic discharges >2.5Hz; non-generalized periodic patterns (e.g. lateralized periodic discharges, bifrontal periodic discharges, etc); non-periodic epileptiform discharges; and non-epileptiform activity. We considered each EEG state to be defined by a combination of background and superimposed findings (Supplemental Table 1). We time-stamped each change in EEG state to the nearest minute. To minimize multiple hypothesis testing, we binned EEG subtypes to test the effect of AEDs on the probability of state transition from epileptiform states to non-epileptiform states. In this aggregated system, we grouped EEG activity into 5 categories: suppressed; discontinuous with superimposed epileptiform activity; discontinuous without epileptiform features; continuous with superimposed epileptiform activity; and continuous without epileptiform features (Supplemental Table 1).
We queried our electronic medical record to generate a report of all AEDs administered to these patients with associated administration timestamps. Timestamps started when the bedside nurse scanned the medication and patient identification band during administration. We considered the following as intermittently dosed AEDs: levetiracetam, valproate, (fos)phenytoin, lacosamide, phenobarbital and enteric clonazepam or alprazolam. Once initiated, we rarely stopped intermittent AEDs during ongoing EEG monitoring, dosing each at a frequency sufficient to presume lasting therapeutic effect between intermittent doses. Thus, we considered these AEDs to be absent up to the time of first administration, then present for the duration of the remaining EEG record. Continuously infused AEDs in this cohort were: propofol, ketamine and benzodiazepines (midazolam and lorazepam). Based on pharmacokinetics, we considered propofol to be present only while actively infusing, then absent if the infusion were discontinued. We could not differentiate from this dataset whether propofol was administered for routine sedation during temperature management, as an AED, or both. Ketamine is longer acting and was rarely discontinued with during EEG monitoring, so we considered present continuously once the infusion was started. In the case of intravenous benzodiazepines, both intermittent intravenous bolus doses (lorazepam, midazolam and diazepam) and as continuous infusions (lorazepam and midazolam) existed. We considered intravenous boluses doses to have a 60-minute duration of effect. Based on prolonged terminal half-life after sustained infusion, we considered the effect of continuous infusions to persist for the duration of EEG monitoring. Finally, to determine the association of targeted temperature management with state transitions, we tested core body temperature as a continuous predictor in our models.
Statistical analysis
We used descriptive statistics to summarize population characteristics and outcomes, and report means with standard deviation and counts with corresponding percentages. Next, we used a multi-state model to summarize the characteristics of EEG state transitions in our cohort irrespective of concurrent AED therapy. To summarize the state transitions in our data, we first built a stationary Markov model that treated transition intensities as constant over time. This model yielded a matrix of transition intensities, which we used to build a transition probability matrix.16–18 Rows in the transition probability matrix correspond to each patient’s current EEG state, while columns correspond to all possible next states to which the EEG might transition after a specified time period. Other useful quantities calculated from the intensity matrix are the mean sojourn times in each state (i.e. the expected duration of a stay in a state), the total expected amount of time spent in each state and the expected number of visits to a particular state during the duration of the monitoring. We report estimates of these quantities, obtained using the R-package msm.19 We measured goodness of fit of this multi-state model by estimating the observed numbers of individuals occupying a state during the first 72 hours and plotting these against forecasts from the fitted model.
Next, we tested whether AEDs alter the transition intensities for movement from epileptiform EEG states (discontinuous background with superimposed epileptiform activity or continuous background with superimposed epileptiform activity) to non-epileptiform EEG states (discontinuous or continuous background without any superimposed epileptiform activity) during the first 72 hours of EEG monitoring. We used Aalen’s linear model, a nonparametric intensity regression method that allows both time dependent effects and time varying covariates using a custom-modified version of the R-package Addreg.20–22 This model can account for time since cardiac arrest, baseline covariates or demographic factors, and time-varying covariates (e.g. AED administration), all of which might be expected to affect the intensity of a future EEG state transition. In contrast to the stationary Markov model, Aalen’s model allows transition intensities to vary over time. Aalen’s model is:
| (1) |
where αrs(t) is the intensity of a transition from state r to state s for a patient in state r at time t. X1(t), …, Xk(t) represent the numerical value of the covariates, which we considered to take a value of 1 if the AED being tested has been administered or 0 if the AED being tested has not been administered. Regression coefficients β1(t), …, βk(t) describe how the covariate influence the intensity of state transition at time t, and β0(t) is the baseline transition intensity for the average patient. A negative value of the regression coefficient for an AED covariate suggests that the presence of the drug decreases the likelihood that the transition in question occurs after AED administration compared to the likelihood in the absence of the AED, while a positive coefficient indicates that the likelihood of the transition is higher after drug administration.
We also adjusted for Pittsburgh Cardiac Arrest Category (PCAC). PCAC is a validated 4-level ordinal measure of global post-arrest illness severity.23,24 Briefly, PCAC I patients are awake and do not undergo EEG monitoring in our system. PCAC II and III patients are comatose with preservation of brainstem reflexes, with and without cardiopulmonary failure, respectively, while PCAC IV patients are deeply comatose with loss of at least some brainstem reflexes and/or no motor response. We individually tested the association of each AED with the probabilities of transitioning from epileptiform states to non-epileptiform.
Aalen’s model allows regression parameters to vary over time which is important in the current setting as administration of an AED after 1 hour of monitoring may have a greater effect on transition intensity than the same AED administered 48 hours later. In the model estimation procedure cumulative regression parameters are calculated, i.e. estimates of . We generated plots of these time-varying cumulative regression parameter estimates with corresponding 95% confidence bands. In these plots, a positive slope means drug administration is associated with an increase in the intensity of state transition, while a negative slope means AED administration is associated with a decrease in the intensity of state transition. We also report the cumulative regression coefficients with corresponding confidence intervals. As a sensitivity analysis we limited our cohort to only those who had at least 24 hours of data and compared our results to ensure that our findings were not reflective of non-random missing data or attrition.
Finally, for AEDs significantly associated with resolution of epileptiform EEG states, we tested their effect as second-line agents. To do this, we considered patients’ data only after administration of the first AED and calculated regression coefficients for the second AED administered. For example, a coefficient for valproate in a model predicting transition from a continuous background with epileptiform activity to a continuous background without epileptiform activity would be calculated conditional on prior levitiracetam administration. A significant positive coefficient in this model indicates that among patients that already received levitiracetam as a first line agent, subsequent treatment with valproate is associated with a further increase the probability of transitioning to a non-epileptiform state.
Results
During the study period, we cared for 1,860 patients resuscitated from cardiac arrest. Of these, 412 were awake and did not undergo EEG monitoring, an additional 241 were excluded for early limitations of care, rearrest or moribund status, and 72 had severe trauma or a primary neurological event. A further 402 did not have available EEG (307) or medication (98) data available, leaving 828 subjects included in our multistate EEG models and 730 in additive regression models. Overall, mean (SD) age of included subjects was 57 (17) years, 312 (38%) were women, and 679 (82%) had initial loss of pulses out-of-hospital, of whom 520 (77%) regained spontaneous circulation in the prehospital setting (Table 1). Most (72%) had an initial non-shockable rhythm, and mean arrest duration was 21 minutes. The overall duration of EEG monitoring was 1785 days, with a median of 40 [IQR 23 to 64] hours per subject. Survival to hospital discharge was 30% and the most common proximate cause of death was withdrawal for perceived poor neurological prognosis.
Table 1.
Baseline patient clinical characteristics and outcomes.
| Clinical characteristic | Overall cohort (n = 828) |
|---|---|
| Age, years | 57 ± 17 |
| Female sex | 312 (38) |
| Arrest characteristics | |
| Out-of-hospital arrest location | 679 (82) |
| Initial arrest rhythm | |
| Ventricular tachycardia/fibrillation | 232 (28) |
| Pulseless electrical activity | 302 (36) |
| Asystole | 222 (27) |
| Unknown | 72 (9) |
| Witnessed collapse^ | 434 (64) |
| Layperson cardiopulmonary resuscitation^ | 170 (25) |
| Prehospital return of pulses^ | 520 (77) |
| Number of epinephrine doses administered | 3 ± 2 |
| Arrest duration, minutes | 21 ± 15 |
| Pittsburgh Cardiac Arrest Category | |
| II | 219 (26) |
| III | 85 (10) |
| IV | 524 (63) |
| Outcomes | |
| Survived to hospital discharge | 248 (30) |
| Proximate cause of death* | |
| Withdrawal for non-neurological reasons | 50 (9) |
| Brain death | 58 (10) |
| Rearrest or multisystem organ failure | 128 (22) |
| Withdrawal for neurological prognosis | 344 (59) |
Data are presented as mean ± standard deviation or raw number with corresponding percentages.
Percentages are reported for the subgroup of out-of-hospital cardiac arrests
Percentages are reported for the subgroup of non-survivors
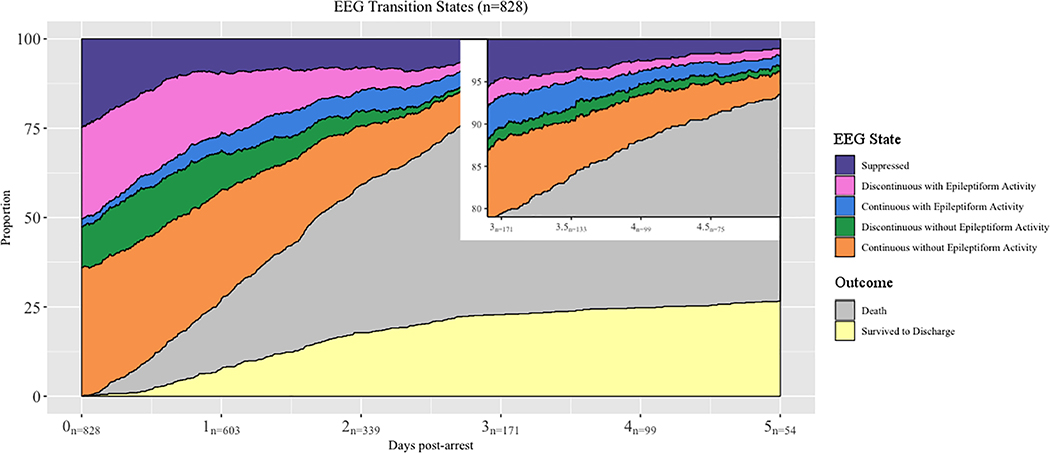
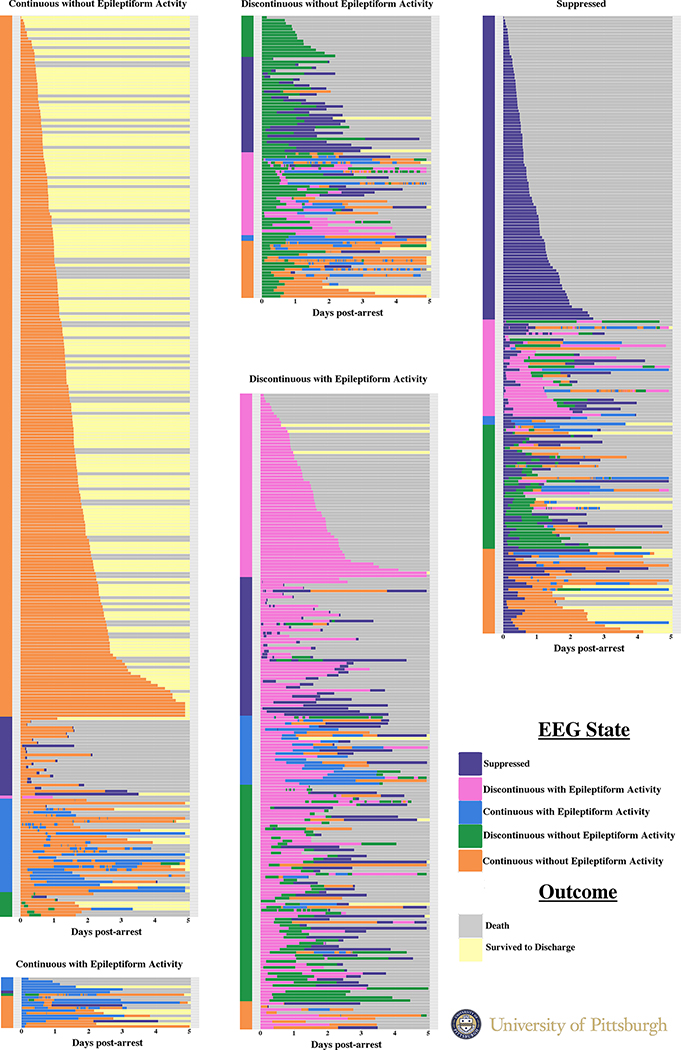
The distribution of initial EEG states is in Table 2, and the frequency with which we observed each EEG state over time is shown in Figure 1. Overall, among patients with epileptiform findings on initial monitoring, 50% transitioned at least once to a non-epileptiform, non-suppressed state (Figure 2). By contrast, 19% with non-epileptiform initial activity transitioned to an epileptiform state at least once. A granular depiction of the disaggregated state transitions for each patient is in Supplemental Figure 1, along with the overall incidence and duration of each EEG state (Supplemental Table 3). Transition probabilities for the next state are presented in Supplemental Figure 2 and reflect the probability of each EEG state being followed by each other EEG state.
Table 2.
Distribution of first electroencephalographic pattern observed upon initiation of monitoring.
| Electroencephalographic states | Overall cohort (n = 828) |
|---|---|
| Background | |
| Suppressed | 205 (25) |
| Suppression-burst | 306 (37) |
| Continuous with periods of attenuation | 106 (13) |
| Continuous | 211 (25) |
| Superimposed patterns | |
| Generalized suppression | 205 (25) |
| Seizures | 7 (1) |
| Polyspike-burst with myoclonus | 180 (22) |
| Polyspike-wave discharges without myoclonus | 25 (3) |
| Generalized periodic discharges ≤2.5Hz | 13 (2) |
| Generalized periodic discharges >2.5Hz | 0 (0) |
| Focal periodic discharges | 5 (1) |
| Non-periodic epileptiform discharges | 49 (6) |
| Non-epileptiform activity | 344 (42) |
Data are presented as raw number with corresponding percentages.
Figure 1.
The frequency of each observed EEG state over time. The number of subject still undergoing monitoring each day is noted along the X axis. The inset highlights enlarges the observed states in patients still undergoing monitoring on Days 3, 4 and 5.
Figure 2.
Heatmap depicting each subject’s electroencephalography interpretation over the first 5 days of continuous EEG monitoring. Each subject’s data constitutes a single bar with the subject’s outcome at hospital discharge following the EEG data. Subjects are grouped by the first observed pattern, then sorted by the second observed pattern (if any).
Overall, 568 (78%) received at least one AED (Table 3). Most common first line agents were propofol and benzodiazepines (Supplemental Table 4), although we could not determine retrospectively the intent with which these were administered (as sedatives or AEDs). Of 345 patients that developed epileptiform EEG activity, 325 (94%) received at least one intermittent AED. Patients who received AEDs without epileptiform activity fell broadly into 3 categories: 1) patients receiving pre-arrest AEDs that were continued in the post-arrest period; 2) AEDs initiated for myoclonic jerks observed clinically before EEG but without epileptiform activity on the subsequent EEG; and 3) equivocal EEGs interpreted clinically as potentially epileptiform but adjudicated by our research team as non-epileptiform.
Table 3.
Frequency and dosing of antiepileptic drugs administered during electroencephalographic monitoring.
| Antiepileptic drug | Number of patients treated (n = 731) | Dosage* |
|---|---|---|
| Continuous infusions | ||
| Benzodiazepines | 261 | 4.7 ± 3.8 |
| Midazolam | 255 | 4.8 ± 3.8 |
| Lorazepam | 11 | 2.2 ± 2.2 |
| Propofol | 587 | 19 ± 11 |
| Ketamine | 60 | 41 ± 32 |
| Phenobarbital | 4 | 26 ± 26 |
| Intermittent agents | ||
| Levetiracetam | 321 | 1000 [750, 1500] |
| Valproate | 205 | 750 [500, 1000] |
| Benzodiazepines | 436 | 2 [1,2] |
| Lorazepam | 218 | 2 [1, 2] |
| Midazolam | 306 | 2 [2, 2] |
| Clonazepam | 25 | 0.5 [0.5, 1] |
| Diazepam | 16 | 20 [5, 20] |
| Alprazolam | 6 | 0.5 [0.5, 0.5] |
| Phenytoin | 110 | 100 [100, 150] |
| Lacosamide | 36 | 100 [100, 150] |
| Phenobarbital | 30 | 510 [100, 922.5] |
| Ketamine | 40 | 70 [40, 100] |
Treatment frequency data are presented as raw number of patients with corresponding percentages. Dosage administration data is presented as the total hours of administration, with the median duration per treated patient, for continuous infusions, and the total number of doses with median doses per treated patient for intermittently dosed antiepileptic drugs.
Continuous infusion dosages are presented as mean ± standard deviation in units milligrams per hour, except propofol which is expressed as micrograms per kilogram actual body weight per minute. Intermittent agent dosages are presented as median [interquartile range] in units of milligrams per dose.
Cumulative baseline transition intensities and the impact of AED administration on these intensities, adjusted for PCAC status, are in Table 4 with time-dependent associations plotted in Supplemental Figure 3. In unadjusted analysis, several individual AEDs were associated with a change in the probability of state transition from an epileptiform EEG state to non-epileptiform states. Among patients with continuous EEG background and epileptiform activity, valproate (β coefficient 2.49 [95% CI 1.38 – 3.61]) and levetiracetam (β coefficient 0.52[95% CI −0.35 – 1.39])were associated with resolution of epileptiform activity. Warmer body temperature was associated with a decrease in the probability of resolution of epileptiform activity in these patients. By contrast, among patients with discontinuous EEG background, no AED was associated with resolution of epileptiform activity, nor was core body temperature. A sensitivity analysis limited to only those patients with > 24 hours of EEG monitoring demonstrated stable findings in this subgroup.
Table 4.
Summary of the dynamics of electroencephalographic states after cardiac arrest during continuous monitoring. The top portion of the table reports the estimated cumulative baseline intensities for clinically relevant state transitions. These are derived from the multistate model and represent the expected number of transitions an average patient will have during the observation period. The bottom portion of the table provides the cumulative regression coefficients from univariable additive regression models adjusted for PCAC status. The cumulative coefficients used for testing the association of each antiepileptic drug (AED) with the change in state transition intensity are calculated over the first 72 hours of monitoring. This represents the impact of AEDs on the observed probability of state transition.
| Parameter | Transition | |||||||
|
From: Discontinuous + epileptiform To: Discontinuous not epileptiform |
From: Discontinuous + epileptiform To: Continuous not epileptiform |
From: Continuous + epileptiform To: Discontinuous not epileptiform |
From: Continuous + epileptiform To: Continuous not epileptiform |
|||||
| Cumulative baseline intensity (95% CI) | Cumulative baseline intensity (95% CI) | Cumulative baseline intensity (95% CI) | Cumulative baseline intensity (95% CI) | |||||
| Expected number of transitions | 1.16 (0.88, 1.35) | 0.29 (0.14, 0.43) | 0.31 (−0.11, 0.74) | 1.53 (1.09, 1.96) | ||||
| Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |
| Medication effects | ||||||||
| Benzodiazepines | −0.7 (−1.42, 0.02) | 0.011 | −0.45 (−0.93, 0.03) | 0.016 | −0.11 (−0.36, 0.15) | 0.674 | 0.19 (−0.63, 1) | 0.550 |
| Propofol | −0.22 (−0.75, 0.31) | 0.750 | 0.08 (−0.2, 0.37) | 0.933 | 0.3 (−0.13, 0.72) | 0.156 | −0.05 (−0.87, 0.78) | 0.550 |
| Ketamine | −0.01 (−1.27, 1.25) | 0.009 | −0.21 (−0.42, 0) | 0.546 | −0.21 (−0.54, 0.13) | 0.175 | −1.51 (−2.66, −0.35) | 0.001 |
| Valproate | −0.39 (−1.06, 0.27) | 0.544 | −0.22 (−0.55, 0.1) | 0.006 | 0.94 (−1, 2.88) | 0.480 | 2.49 (1.38, 3.61) | 0.000 |
| Levetiracetam | −0.62 (−1.74, 0.5) | 0.970 | −0.04 (−0.36, 0.28) | 0.274 | −0.27 (−0.68, 0.14) | 0.172 | 0.52 (−0.35, 1.39) | 0.019 |
| Phenytoin | −0.42 (−0.94, 0.11) | 0.048 | −0.36 (−0.6, −0.12) | 0.002 | 0 (0, 0) | 0.237 | −0.75 (−1.58, 0.07) | 0.012 |
| Phenobarbital | −0.22 (−0.91, 0.47) | 0.412 | −0.24 (−0.51, 0.03) | 0.172 | 0 (0, 0) | 0.317 | 0.91 (−0.77, 2.58) | 0.233 |
| Lacosamide | −0.45 (−1.31, 0.42) | 0.932 | −0.38 (−0.6, −0.16) | 0.000 | 0 (0, 0) | 0.286 | −0.82 (−1.26, −0.39) | 0.000 |
| Core temperature (°C−1) | 0.05 (−0.18, 0.26) | 0.658 | 0.02 (−0.10, 0.13) | 0.379 | −0.05 (−0.11, 0.02) | 0.279 | −0.30 (−0.78, 0.17) | 0.042 |
Discussion
There are three plausible explanations for the well-established association between epileptiform EEG patterns and outcome. First, epileptiform EEG activity may be an epiphenomenon of severe primary anoxic injury, and epileptiform patterns neither cause secondary injury nor respond to antiepileptic drug (AED) treatment.25 Second, epileptiform EEG activity might reflect severe primary injury, contribute to secondary injury,26–29 but not respond to AEDs. Finally, epileptiform EEG activity may cause secondary brain injury26–29 and improve with AED treatment.25,30 In the first two scenarios, EEG would have prognostic value, but AED administration would not be expected to affect the EEG or patient outcomes. Only in the third scenario would AED treatment be expected to improve outcomes by promoting resolution of injurious EEG activity. Continued debate about the value of administering AEDs after cardiac arrest demonstrates the need to distinguish between these scenarios.
Our present findings are consistent with prior qualitative reports showing that many highly epileptiform EEG patterns that develop on an otherwise suppressed background, particularly burst suppression with identical bursts, evolve in a stereotyped manner and may be refractory to AED therapy.12,13,25 The ability to generate continuous cortical background activity implies the presence of functional cortical neurons with some degree integrity of both the cortical network and connectivity with deep brain structures. After severe injury, deafferentation and laminar necrosis can result in a suppression-burst pattern.13,14,31,32 Patients with discontinuous background activity and epileptiform EEG activity may lack sufficient cortical substrate to respond to AEDs.
Our data support a potential utility of valproate and levetiracetam for treatment of epileptiform EEG activity in selected comatose post-arrest patients. Use of AEDs to treat epileptiform EEG activity in critically ill patients with acute brain injury is largely extrapolated from management of outpatient epilepsy or convulsive status epilepticus33 despite these being fundamentally different patient populations. Providers administer AEDs with the hope of controlling epileptiform activity and thereby reduce secondary brain injury, a posit with face validity.26–29,34 Small prior studies have reported that AEDs are well tolerated in the critically ill and that seizures often stop after AED administration.35,36 However, prior studies have not adjusted for the probability of spontaneous state transition in the absence of AED treatment. We demonstrate that spontaneous resolution of epileptiform activity is common in this population, a potential threat in assessing impact in a non-experimental design. The ongoing randomized Treatment of ELectrographic STatus Epilepticus After cardiopulmonary Resuscitation (TELSTAR) study will shed further light on the efficacy of AEDs after cardiac arrest.37 Our finding that lower body temperature is also associated with an increase in the probability of resolution of epileptiform activity in patients with continuous background activity is also consistent with prior studies demonstrating a reduction in sustained electrographic status epilepticus38 among hypothermia-treated patients presenting with convulsive status epilepticus. Our results suggest continuity of the EEG background activity is a measureable source of heterogeneity that may allow identification of subgroups of patients unlikely to respond to treatment.
Our study is limited in many ways. First, EEG monitoring started on ICU admission, not immediately post-arrest. At our center, approximately two out of three patients have initial care at another facility; we previously reported a delay of 6 to 8 hours from arrest to initiation of EEG monitoring.9 Thus, we cannot exclude the possibility of clinically important EEG state transitions or differential drug effect in the immediate post-arrest period. Time to AED administration is a potential factor in seizure control,39 so our failure to observe an association between AED administration and resolution of epileptiform activity in patients with discontinuous EEG backgrounds may simply reflects excessive delay to treatment. Second, we decided to combine patterns on the ictal-interictal spectrum to avoid multiple hypothesis testing, since any EEG state can theoretically transition to any other state and more granular treatment of the EEGs would have resulted in several thousand coefficients from our regression models. We cannot comment on between-pattern heterogeneity in drug responsiveness (e.g. generalized periodic discharges and seizures may not respond identically to a given AED). We did not observe an association between benzodiazepines or propofol and transition from continuous to discontinuous background states. In this observational study, we could not determine retrospectively whether these medication were administered to provide sedation or as AEDs, and average dosing was well below recommended anticonvulsant doses. Thus, the lack of observed association of these medications with EEG transitions may simply reflect subtherapeutic dosing.
It is also important to note that clinicians who decided which and how many AEDs to administer were not blinded to other clinical characteristics. Thus, AEDs may have been administered differentially to patients based on perceived severity of brain injury or extracranial organ failure. Sicker patients may have tended to receive more, fewer or different AEDs in difficult to measure ways. Because illness severity and development of epileptiform EEG patterns after cardiac arrest are correlated, observational data cannot fully account for this potential confounder. Additionally, our observational design included patients with variable duration of monitoring. We studied the shape of the cumulative regression coefficient plots derived from the linear model to ensure that significant results yielded slopes that were largely stable over time and were not simply reflective of non-random missing data or attrition. Finally, our first-line intermittent AEDs in this population historically are levetiracetam and valproate, the same AEDs observed to have potential impact. The lack of apparent phenytoin effect may reflect that it is typically administered to patients that have already failed to respond to one or two other AEDs and that past refractoriness to treatment predicts future refractoriness. Thus, we are cautious to interpret negative coefficients to indicate a lack of demonstrable efficacy, rather than that the drug in question actually decreases the probability transitioning to a non-epileptiform state. An experimental design based on these hypotheses generating data seems the next best step to better detect AED impact after cardiac arrest.
In conclusion, we observed that antiepileptic drug administration increases the probability that epileptiform EEG activity resolves after cardiac arrest in the subset of patients able to generate continuous EEG background activity. By contrast, our data suggest that epileptiform EEG activity superimposed on a suppressed background may reflect diffuse cortical damage that and may not be amenable to AED treatment.
Supplementary Material
Acknowledgments
Disclosures: Dr. Elmer’s research time is supported by the NIH through grants 5K12HL109068 and 1K23NS097629.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Daya MR, Schmicker RH, Zive DM, et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC). Resuscitation. 2015;91:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. The New England journal of medicine. 2012;367(20):1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amorim E, Rittenberger JC, Baldwin ME, Callaway CW, Popescu A, Post Cardiac Arrest S. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossetti AO, Tovar Quiroga DF, Juan E, et al. Electroencephalography Predicts Poor and Good Outcomes After Cardiac Arrest: A Two-Center Study. Critical care medicine. 2017;45(7):e674–e682. [DOI] [PubMed] [Google Scholar]
- 7.Westhall E, Rossetti AO, van Rootselaar AF, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86(16):1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmer J, Gianakas JJ, Rittenberger JC, et al. Group-Based Trajectory Modeling of Suppression Ratio After Cardiac Arrest. Neurocritical care. 2016;25(3):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh SH, Park KN, Kim YM, et al. The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84(2):200–205. [DOI] [PubMed] [Google Scholar]
- 11.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40(10):2867–2875. [DOI] [PubMed] [Google Scholar]
- 12.Elmer J, Rittenberger JC, Faro J, et al. Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Annals of neurology. 2016;80(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmeijer J, Tjepkema-Cloostermans MC, van Putten MJ. Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2014;125(5):947–954. [DOI] [PubMed] [Google Scholar]
- 14.van Putten M, Jansen C, Tjepkema-Cloostermans MC, et al. Postmortem histopathology of electroencephalography and evoked potentials in postanoxic coma. Resuscitation. 2018. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27. [DOI] [PubMed] [Google Scholar]
- 16.Jackson C Multi-state modelling with R: the msm package. Cambridge, UK 2007.
- 17.R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 18.Skogvoll E, Eftestol T, Gundersen K, et al. Dynamics and state transitions during resuscitation in out-of-hospital cardiac arrest. Resuscitation. 2008;78(1):30–37. [DOI] [PubMed] [Google Scholar]
- 19.Jackson C Multi-State Models for Panel Data: The msm Package for R. 2011 %9 %! Multi-State Models for Panel Data: The msm Package for R. 2011;38(8 %@ 1548–7660 %8 2011–01-04 %7 2011–01-04):28. [Google Scholar]
- 20.Aalen OO, Fosen J, Weedon-Fekjaer H, Borgan O, Husebye E. Dynamic analysis of multivariate failure time data. Biometrics. 2004;60(3):764–773. [DOI] [PubMed] [Google Scholar]
- 21.Kvaloy JT, Skogvoll E, Eftestol T, et al. Which factors influence spontaneous state transitions during resuscitation? Resuscitation. 2009;80(8):863–869. [DOI] [PubMed] [Google Scholar]
- 22.Aalen OO. A linear regression model for the analysis of life times. Stat Med. 1989;8(8):907–925. [DOI] [PubMed] [Google Scholar]
- 23.Coppler PJ, Elmer J, Calderon L, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82(11):1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rundgren M, Westhall E, Cronberg T, Rosen I, Friberg H. Continuous amplitude- integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Critical care medicine. 2010;38(9):1838–1844. [DOI] [PubMed] [Google Scholar]
- 26.Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol. 2017;74(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Annals of neurology. 2016;79(4):579–590. [DOI] [PubMed] [Google Scholar]
- 28.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Annals of neurology. 2013;74(1):53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claassen J, Albers D, Schmidt JM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Annals of neurology. 2014;75(5):771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72(8):744–749. [DOI] [PubMed] [Google Scholar]
- 31.Niedermeyer E, Sherman DL, Geocadin RJ, Hansen HC, Hanley DF. The burst-suppression electroencephalogram. Clin Electroencephalogr. 1999;30(3):99–105. [DOI] [PubMed] [Google Scholar]
- 32.Thomke F, Brand A, Weilemann SL. The temporal dynamics of postanoxic burst-suppression EEG. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2002;19(1):24–31. [DOI] [PubMed] [Google Scholar]
- 33.Husain AM. Treatment of Recurrent Electrographic Nonconvulsive Seizures (TRENdS) study. Epilepsia. 2013;54 Suppl 6:84–88. [DOI] [PubMed] [Google Scholar]
- 34.Husain AM. Lacosamide in status epilepticus: Update on the TRENdS study. Epilepsy Behav. 2015;49:337–339. [DOI] [PubMed] [Google Scholar]
- 35.Swisher CB, Doreswamy M, Husain AM. Use of pregabalin for nonconvulsive seizures and nonconvulsive status epilepticus. Seizure. 2013;22(2):116–118. [DOI] [PubMed] [Google Scholar]
- 36.Nau KM, Divertie GD, Valentino AK, Freeman WD. Safety and efficacy of levetiracetam for critically ill patients with seizures. Neurocritical care. 2009;11(1):34–37. [DOI] [PubMed] [Google Scholar]
- 37.Ruijter BJ, van Putten MJ, Horn J, et al. Treatment of electroencephalographic status epilepticus after cardiopulmonary resuscitation (TELSTAR): study protocol for a randomized controlled trial. Trials. 2014;15:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legriel S, Lemiale V, Schenck M, et al. Hypothermia for Neuroprotection in Convulsive Status Epilepticus. The New England journal of medicine. 2016;375(25):2457–2467. [DOI] [PubMed] [Google Scholar]
- 39.Claassen J, Goldstein JN. Emergency Neurological Life Support: Status Epilepticus. Neurocritical care. 2017;27(Suppl 1):152–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.