Fig. 2.
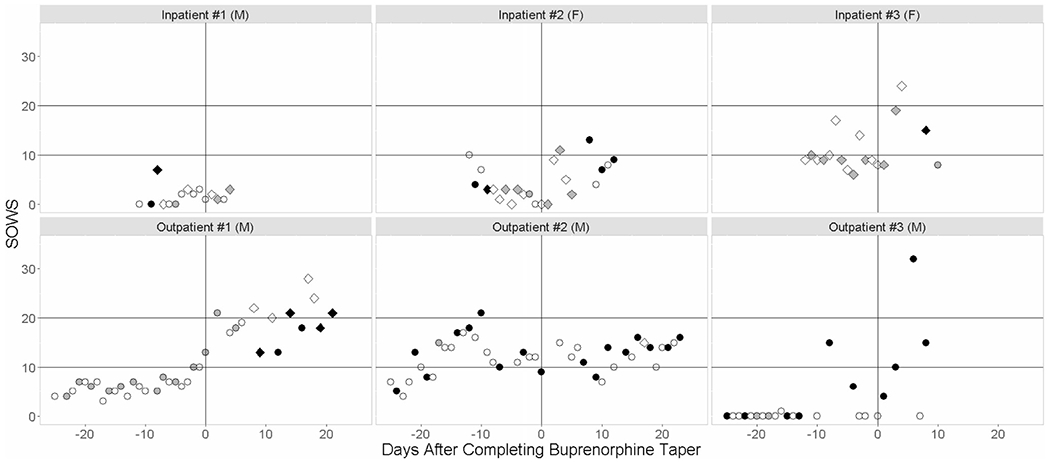
Time trends in SOWS scores with rescue-medication use and urine opioid results for participants receiving pioglitazone (3 inpatients, 3 outpatients). The time points in the figure represent the portion of the study during which participants were receiving pioglitazone. The duration of the buprenorphine taper was 28 days for participants enrolled in the initial outpatient study design, and 13 days for participants enrolled in the subsequent inpatient study design. The color of the marker indicates opioid positivity (positive = black, negative = gray, not tested = white) while marker shape indicates use of adjunct medications (diamond = yes, circle = no). Participant sex (F = female, M = male) is indicated in parentheses
