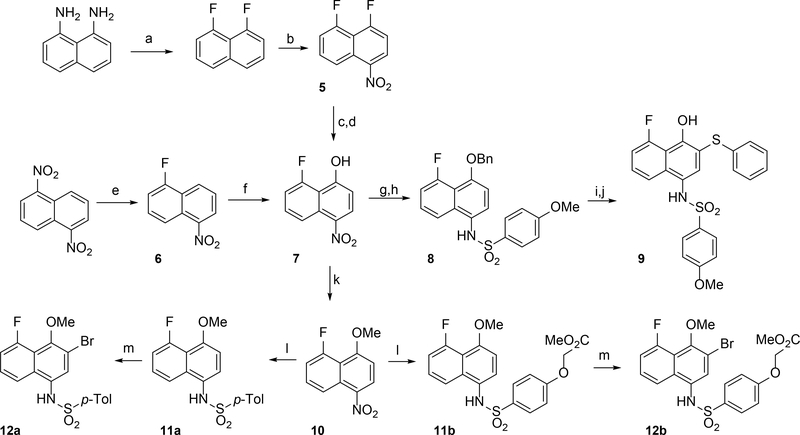
Scheme 2.
Synthesis of 5-fluoronaphthylsulfonamides (a) i) HBF4 (aq) NaNO2 ii) molten KHF2 (2–20%) (b) HNO3, NaNO2 (60%) (c) BnOH, NaH, CH2Cl2 (61%) (d) Pt/C, NaBH4 (99%) (e) CsF, DMSO, 100 °C (23%) (f) NH3, TBHP, NaOH, THF (70%) (g) BnBr, Cs2CO3, CH2Cl2 (75%) (h) i) Pt/C, NaBH4 ii) 4-methoxybenzenesulfonyl chloride, pyridine, MgSO4 (62%) (i) Pd/C, H2, (j) PhI(OAc)2, PhSH, (CF3)2CHOH (37%) (k) MeI, K2CO3, DMF (84%) (l) i) Pd/C, NaBH4 ii) R2SO2Cl, pyridine, MgSO4 (45–89%) (m) Br2, CH2Cl2 (56–75%)