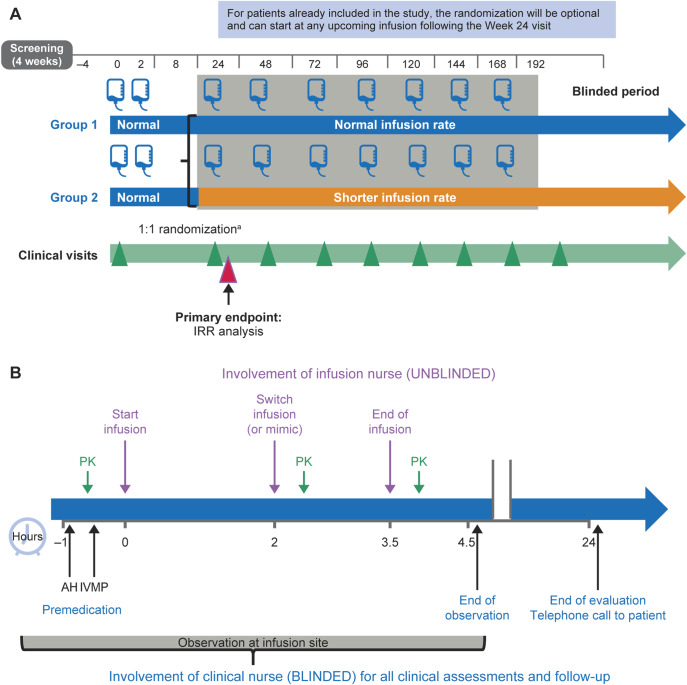
Figure 1. ENSEMBLE PLUS (A) study design and (B) infusion schedule.
The ENSEMBLE PLUS primary end point is the proportion of patients with IRRs after the first randomized dose (frequency and severity assessed during and 24 hours postinfusion). aRandomization of new patients at week 24. AH = antihistamine; IRR = infusion-related reaction; IVMP = methylprednisolone; PK = pharmacokinetic assessment.