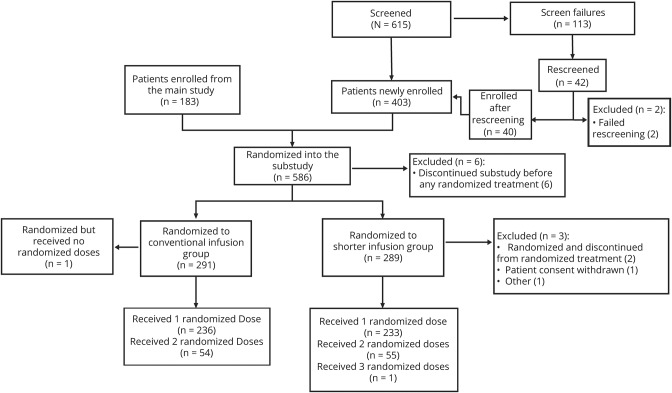
Figure 2. Patient disposition and analysis of population.
One patient disclosed that they were pregnant after randomization but before receiving any study treatment. Per protocol, treatment is withheld from patients who become pregnant during the study. There was also one withdrawal from the conventional infusion group because of an adverse event (depressive symptom) that was considered unrelated to the study treatment but because of concurrent illness of depression. A discontinuation visit had not been scheduled or undertaken for the patient at the time of CCOD; hence, this patient could not be included in any of the tables which display discontinuation. Other: accidental unblinding. CCOD = clinical cutoff date.