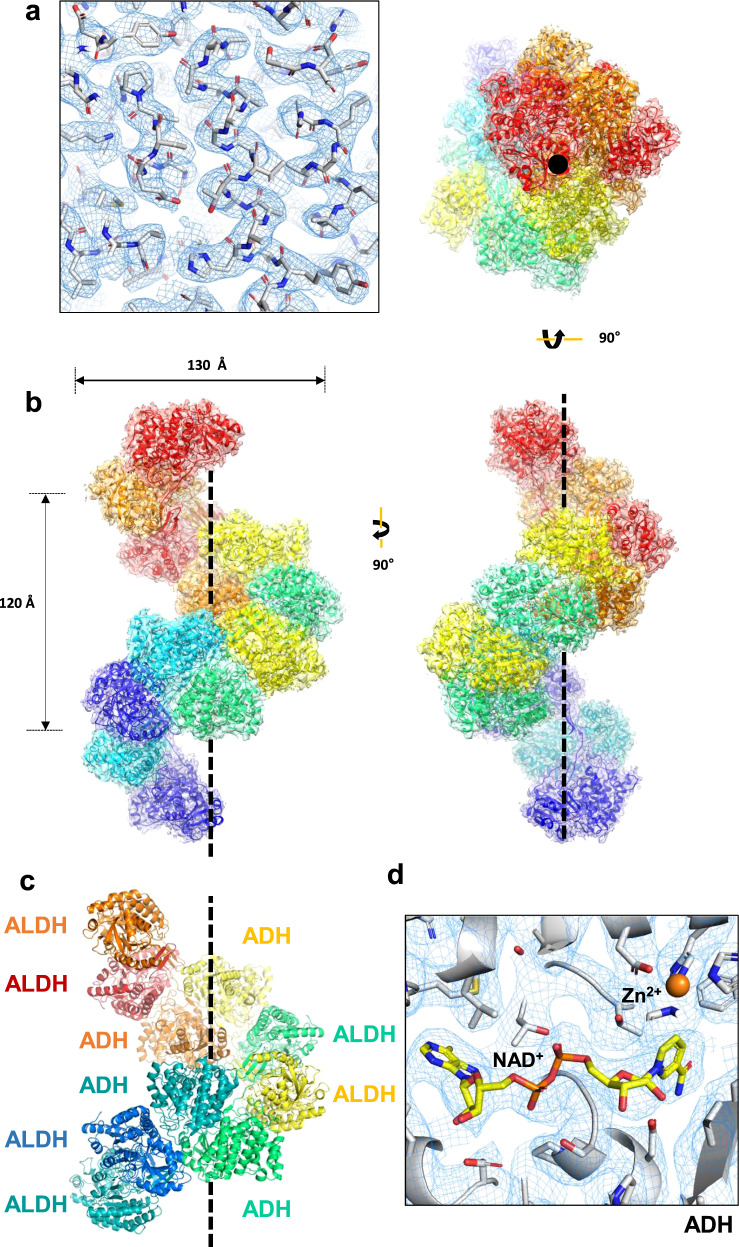
Fig. 2. The cryo-EM structure of the AdhE spirosome in an extended conformation.
a The cryo-EM map fitted with the atomic model. b The atomic model of AdhE in an extended conformation in three different views. AdhE forms spirosomes with a width of 130 Å and four AdhE molecules form one helical turn with a pitch of 120 Å. AdhE molecules are labeled in different colors. c One helical turn with one ALDH domain at the near bottom (blue) and one ADH domain at the near top (red) is shown. One helical turn is composed of four AdhE molecules (each AdhE is colored in orange, yellow, green, and cyan). An AdhE dimer is formed by intertwining ALDH and ADH domains, and a tetramer AdhE is formed majorly by ADH–ADH interaction in a tail-to-tail manner. d The cryo-EM map shows clear density for NAD+ and Zn2+ near the catalytic pocket of the ADH domain.