Abstract
Increased availability of cannabis and cannabinoid-containing products necessitates the need for an understanding of how these substances influence aging. In this study, zebrafish (Danio rerio) were exposed to different concentrations of THC (0.08, 0.4, 2 μM) during embryonic-larval development and the effects on aging were measured 30 months later and in the offspring of the exposed fish (F1 generation). Exposure to 0.08 μM THC resulted in increased male survival at 30 months of age. As the concentration of THC increased, this protective effect was lost. Treatment with the lowest concentration of THC also significantly increased egg production, while higher concentrations resulted in impaired fecundity. Treatment with the lowest dose of THC significantly reduced wet weight, the incidence of kyphosis, and the expression of several senescence and inflammatory markers (p16ink4ab, tnfα, il-1β, il-6, pparα and pparγ) in the liver, but not at higher doses indicating a biphasic or hormetic effect. Exposure to THC did not affect the age-related reductions in locomotor behavior. Within the F1 generation, many of these changes were not observed. However, the reduction in fecundity due to THC exposure was worse in the F1 generation because offspring whose parents received high dose of THC were completely unable to reproduce. Together, our results demonstrate that a developmental exposure to THC can cause significant effects on longevity and healthspan of zebrafish in a biphasic manner.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00175-3) contains supplementary material, which is available to authorized users.
Keywords: Cannabinoids, Cannabis, Inflammaging, Senescence
Introduction
In the wake of state-level policy changes, consumer access to cannabis is at an all-time high in the USA. Roughly 20% of young adults between the ages of 18–25 admit to using marijuana in the past month (Ahrnsbrak et al., 2017), and the incidence of cannabis usage in pregnant women, particularly in the first trimester, has more than doubled in the past decade (Volkow et al., 2019). In fact, marijuana use among pregnant women is higher than any other illicit drug (Volkow et al., 2019). Importantly, 70% of both pregnant and nonpregnant women believe there is slight or no risk of marijuana use (Ko et al., 2015). However, there is surprisingly little data on the long-term effects of cannabis exposure during embryonic development. Some studies link marijuana use with adverse birth outcomes including low birthweight and preterm birth (El-Mohandes et al., 2003; Fergusson et al., 2002; Hayatbakhsh et al., 2012), while other studies do not observe any effects (Bada et al., 2005; Desai et al., 2014; van Gelder et al., 2010).
The major psychoactive component and the most common phytocannabinoid in cannabis is Δ9-tetrahydrocannabinol (THC). THC can cross the placenta (Hurd et al., 2005; Hutchings et al., 1989), and its use during pregnancy can alter the cannabinoid signaling pathways within the developing fetus. It is very hard to establish clear effects of THC in humans because of uncertainties associated with: a wide range in exposure concentrations; confounding effects of the other cannabinoids and terpenes found in cannabis; and potential effects of co-exposures to other drugs, alcohol, or environmental stressors. Furthermore, many of the studies in humans focus on short-term effects, while the effect of THC exposure on the processes of aging remains largely unknown. One known long-term consequence of maternal exposure to high doses of THC is the reduction in fertility of male offspring (Dalterio and DeRooij, 1986). Clearly, there is a need to further elucidate the effects of in utero THC exposure particularly on longer term outcomes such as lifespan and healthspan.
It is well-known that early-life stressors can instigate long-term changes in health. This concept is often referred to as the Developmental Origins of Health and Disease (DOHaD), or, with respect to the processes of aging, Developmental Aging theory (DevAge) (Barker, 2007; Feltes et al., 2015). These stressors can be chemical and/or environmental (reviewed in: Haugen et al., 2015), and the effects can be detrimental by increasing the risk of disease (Richardson et al., 2006; Zawia and Basha, 2005), or beneficial by increasing resilience to age-related dysfunction (Calabrese and Mattson, 2017; Gidday, 2015; Hodges and Ashpole, 2019). There is some evidence that THC may increase resilience to certain stressors because preconditioning with low doses of THC protects against a wide range of neuronal insults, including 3,4 methylene-dioxymethamphetamine (MDMA) stress, exposure to carbon monoxide, and other neuronal stressors (Assaf et al., 2011; Fishbein-Kaminietsky et al., 2014; Hodges and Ashpole, 2019). These are acute effects of preconditioning with limited evidence that protection continues in the months following THC exposure. There has been increasing interest by researchers to determine whether THC or other cannabinoids could positively impact neurological health and neurodegenerative disease development in advanced age. Indeed, cannabinoids protect against neurodegenerative diseases in many animal models when they are administered in adulthood or advanced age (Fernández-Ruiz et al., 2017). The anti-inflammatory effects of THC may contribute to some of its protection against neurodegenerative diseases (Ramírez et al., 2005). While high doses of THC can cause memory deficits (Varvel et al., 2001), low dose exposure to THC can slow or halt Alzheimer’s disease progression by reducing amyloid beta (Cao et al., 2014), as well as restoring cognitive function in old mice (Bilkei-Gorzo et al., 2017). Together, with previous findings that high dose exposure to THC in utero causes disrupted brain development and other teratogenic effects (Carty et al., 2018; Fish et al., 2019), it is easy to hypothesize that the dose and the age of exposure determine the beneficial versus detrimental effects of THC on neuronal health.
In this current study, we investigated whether embryonic THC exposure results in later-life, sex-specific changes in behavior, reproduction, body size and weight, metabolism, or molecular markers of senescence and inflammation. We utilized zebrafish as a model for this study due to their extra-uterine and rapid development, high fecundity, and availability of genetic resources. To accomplish this, developmentally exposed fish (embryo-larval) were enrolled into the study at 12 months of age, and the effects of aging were assessed when they were 30 months old. THC was predicted to worsen the age-related loss in locomotor function and fecundity and exacerbate the age-associated increase in kyphosis, senescence, and inflammation. To determine if the observed effects are multigenerational, aged F1 exposed to the highest dose of THC (parents exposed) were also cultured and assessed at 30 months of age.
Methods
Zebrafish care and exposure
All experiments were approved by the University of Mississippi IACUC. As reported in (Pandelides et al., 2020), standard Tg(fli1:egfp) zebrafish were purchased from the Zebrafish International Resource Center (ZFIN, Eugene, Oregon) and bred in house. Fish were maintained in Aquatic Habitats ZF0601 Zebrafish Stand-Alone System (Aquatic Habitats, Apopka, FL) with zebrafish water (pH 7.0–7.5, 60 ppm (ppm) Instant Ocean, Cincinnati, OH) at 25–28 °C, 14:10 light-dark cycle. Fish were fed twice daily with Gemma (Gemma Micro 300, Skretting).
Fertilized embryos were exposed to sub-lethal concentrations (Carty et al., 2019), 0.08, 0.4, 2 μM (0.024, 0.12, 0.6 mg/L) THC, or 0.05% DMSO control water at a 0.6:1 (mL water:fish) ratio from 6 to 96 h post fertilization (hpf). Measured concentrations for 0.6 mg/L THC (nominal) was 0.67 ± 0.05 mg/L THC as reported in (Carty et al., 2019). The NIDA Drug Supply Program supplied all THC used throughout the developmental exposures. Further methodology of exposure and rearing of the F0 and F1 generation are described in Carty et al. (2019). At 12 months of age, F0 (exposed) or F1 (parents exposed) fish were enrolled into the aging study and observed until 30 months of age. This study was conducted simultaneously with the CBD exposure study (concurrent submission), both THC and CBD treated fish were reared in the same room, under the same conditions, and shared the same control fish.
Fecundity and larval endpoints
To determine fecundity in the aged cohorts, two males and two females from each treatment group were placed into static breeding tanks (n = 3 spawning tanks per concentration) and allowed to acclimate for 1 week prior to egg collection. Eggs were then collected for three consecutive days per previous recommendation (Reed and Jennings, 2011), and the average number of eggs collected was quantified. Eggs were transferred to a clean embryo medium (60 ppm Instant Ocean; pH 7.5–7.8) as they developed. The eggs were subsequently observed every 24 h to determine fertilization rate, mortality, malformations, and hatching. Developmental deformities were visually assessed at 96 hpf by anesthetizing the fish in 300 mg/L tricaine methanesulfonate (MS-222) and 600 mg/L sodium bicarbonate, and subsequently imaging a lateral profile with a MicroFire® camera (Optronics, Goleta, CA) attached to a Zeiss Stemi 2000-C Stereo Microscope (Jena, Germany) using the Picture Frame™ Application 2.3 software (Optronics, Goleta, CA). Three double-blinded reviewers scored the presence or absence of developmental abnormalities. Additionally, the total body length, and the size of the eye were calculated on ImageJ (Schneider et al., 2012). After imaging, the fish were removed from anesthesia and returned to their respective tanks until behavioral assessment in the open field test.
Open field behavior
Fish were transferred to a darkened behavioral testing room (27–28 °C) and allowed to acclimate prior to open field behavioral assessment. The open field arena consisted of a water-filled bucket (diameter of 23.5 cm and a depth of 24.8 cm) with overhead LED lighting set to an intensity of 9 lx. Fish were transferred to the arena using a capture net and released into the bucket where they swam freely for 5 min. Their movement was video-captured overhead and analyzed using the Noldus Ethovision 14 software. Distance, swim speed, mobility, and time spent in the center (inner 50% of the arena) and periphery (outer 50% of the arena) was then calculated by Ethovision and decoded by a blinded observer.
Phenotypic assessment of adults
Fish were euthanized in 300 mg/L MS-222 and 600 mg/L sodium bicarbonate, and overall wet weight and length of the fish were recorded. A lateral profile picture was captured for assessment of kyphosis by three double-blinded observers. As reported in our concurrent submission, each rater used a scale of 1–5, with 1 signifying no curvature and 5 signifying severe curvature to assess the extent of kyphosis. Inter-observer scoring showed 76% identity and over 90% similarity. The median score for each fish was determined and used for comparison between groups. Following imaging, necropsies were performed and incidence of gross tumors was recorded.
RT-qPCR
Liver tissue was isolated following euthanasia and homogenized in TRIzol (Invitrogen #A33251). RNA was extracted using an RNeasy mini-kit (Qiagen #74104) in conjunction with gDNA removal via an RNase-Free DNase set (Qiagen #79254) following manufacturer’s recommended protocol, and subsequently quantified and evaluated for purity (260/280 ratio = 1.9–2.1) on a NanoDrop 2000 (Thermo Fisher, Waltham, Massachusetts). RNA (250 ng) was then reverse transcribed to 10 ng/μL cDNA following manufacture’s protocol (Invitrogen kit #4304134). RT-qPCR was performed using an Applied Biosystems 7200 real-time cycler with SYBR Green detection chemistry (Applied Biosystems #4309155) following manufacture’s protocol (25 μL reaction volume). Parameters for RT-qPCR were as follows: 95 °C for 10 min, then 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by 95 °C for 15 s-60 °C for 1 min-95 °C for 15 s dissociation curve. Primers were optimized as in Corrales et al. (2014) and detailed in Supplemental Table 1. Technical duplicates were performed and resulting data was evaluated using the 2-ΔΔCT method (Livak and Schmittgen, 2001) and displayed using the average log(2)ΔΔCt ± standard error of the mean.
Statistical analysis
To compare the effects of aging, differences in young controls vs aged controls were assessed using a t test (p ≤ 0.05). To compare the effects of CBD, differences in the varying doses of CBD vs aged controls were assessed using a one-way ANOVA followed by the post hoc Dunnett’s test against aged control (p ≤ 0.05). Categorical data including kyphosis, yolk sac edema, pericardial edema, and adult survival were analyzed using Chi squared (p ≤ 0.05), or Fisher’s exact test (p ≤ 0.05). All graphing and statistical analysis was conducted using the Sigmaplot 14.0 software.
Results
Locomotor behavior following THC exposure
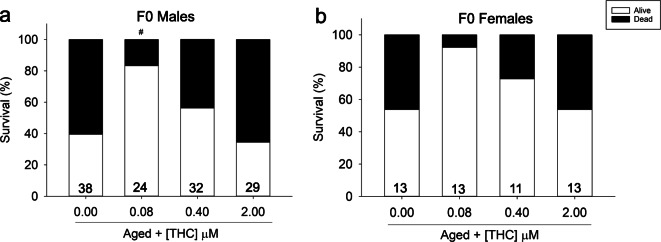
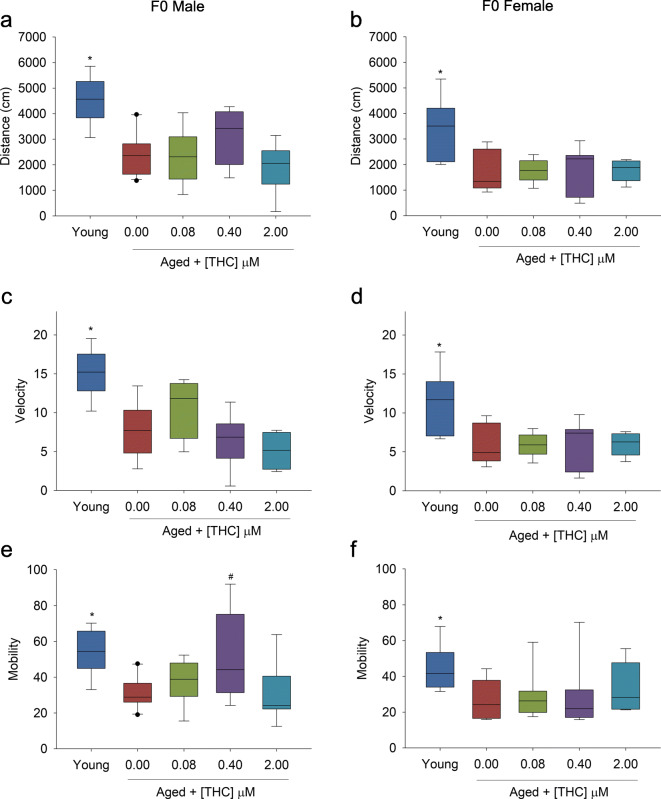
Male and female zebrafish were exposed during embryo to larval development (6–96 hpf) to increasing concentrations of THC (0.08, 0.4, and 2 μM). This exposure was concurrent with the developmental CBD exposure described in the concurrent submission; the controls used are the same for both papers. One year following exposure, fish were enrolled into the longevity study. By 30 months of age, 60% of control male and female fish had died (Fig. 1a, b). THC caused a biphasic effect on survival, with significantly increased survival at 0.08 μM THC (80%), and returning to control levels at 2 μM (40%) in male fish (Fig. 1a). Females treated with 0.08 and 0.4 μM THC early in life had a non-significant trend of increased survival at the 30-month time point. Behavior phenotypes were then assessed in a cross-sectional study at this 30-month time point. Aged zebrafish showed significant reductions in distance moved (Fig. 2b), swim speed (Fig. 2c, d), and overall mobility (Fig. 2e, f) in the open field compared with young, 7-month old control fish. With the exception of increased mobility in 0.4 μM THC-exposed males, early-life treatment with THC did not improve locomotor abilities in aged male or female fish (Fig. 2a–f), even at the concentrations that showed increased survival (Fig. 1). There was no significant effect of developmental THC exposure on time fish spent swimming in periphery or center of the open field arena (data not displayed).
Fig. 1.
Survival (%) measured at 30 months of age of adult F0 zebrafish developmentally exposed to THC and enrolled into the study at 12 months old. a Male % survival from 12 to 30 months, n = 27–38. b Female % survival from 12 to 30 months, n = 7–13. The number displayed at the base of each bar is the total number of fish per treatment enrolled into the study at 12 months old. Number sign indicates a significant difference compared with aged controls (Fisher’s exact test p ≤ 0.05)
Fig. 2.
Open field behavior of adult F0 (young = 7 months old, aged = 30 months old zebrafish developmentally exposed to THC, n = 8. a, b Male and female zebrafish distance traveled (cm). c, d Male and female velocity (cm/s). e, f Male and female mobility (%). Asterisk indicates a significant difference between aged and young controls (t test, p ≤ 0.05). Number sign indicates a significant difference compared with aged controls (ANOVA, Dunnett’s posthoc, p ≤ 0.05)
Fecundity following THC exposure
Late-life fecundity was assessed by quantifying egg and sperm production as well as the survival of fertilized eggs. As described in (Pandelides et al., 2020), aged control male and females produced significantly less sperm and eggs than young controls (Table 1). Early-life exposure to 0.08 and 0.4 μM THC did not significantly alter sperm production, and there were unfortunately not enough extra male fish to determine sperm concentration for 2 μM THC (Table 1). Early-life exposure to THC resulted in a hormetic response with increased egg production as the dose of THC was decreased. Exposure to 0.08 μM THC partially rescued egg production in aged females producing 493% more eggs than the aged controls (Table 1). These offspring had similar 96 hpf survival to both young and aged control fish (53–62% survival, Table 1). However, while fish exposed to 2 μM THC produced a similar number of eggs as aged control fish, only 16% of these survived by 96 hpf (Table 1). Exposure to 0.4 μM resulted in intermediary effects between 2 and 0.08 μM THC, with a non-significant increase in egg production (55% reduced relative to young fish) and a non-significant decrease in survival by 96 hpf. The offspring exposed to 0.08 and 0.4 μM THC displayed increased size at 96 hpf (Supplemental Figure 1A). Conversely, these larvae also had reduced eye diameter with increasing THC concentration (Supplemental Figure 1B). However, these surviving offspring did not exhibit robust abnormalities, as no significant differences in yolk sac or cardiac structure were observed (Supplemental Fig. 1C–G).
Table 1.
F0 fecundity and survival of THC exposed (μM) zebrafish, data presented as mean ± SE
| Nominal developmental treatment (μM) at 6 hpf | n | Avg no. of eggs per tank per week ± SE | % survival at 24 hpf ± SE | % hatch at 48 hpf ± SE | % hatch at 72 hpf ± SE | % survival at 96 hpf ± SE | Sperm/g testes*106 ± SE |
|---|---|---|---|---|---|---|---|
| Young control | 6 | 491 ± 71.7* | 66.2 ± 5 | 48.3 ± 5.3* | 98.5 ± 1.4 | 62.8 ± 4.3 | 31.2 ± 3.1* |
| Aged control | 6 | 73 ± 43 | 59.9 ± 6.6 | 18.3 ± 8.6 | 100 ± 0 | 56.4 ± 7.2 | 13.4 ± 1.5 |
| 0.08 THC | 6 | 360.4 ± 101.0# | 59.4 ± 4.7 | 39.9 ± 16.2 | 97.3 ± 1.9 | 53.2 ± 4.7 | 9.1 ± 1.5 |
| 0.4 THC | 6 | 220 ± 42.1 | 42.1 ± 10.4 | 45.7 ± 14.5 | 95.8 ± 3.6 | 36.8 ± 9.7 | 5.9 ± 4.7 |
| 2 THC | 6 | 92.8 ± 58.7 | 19.7 ± 3.8 | 22 ± 0.5 | 89.9 ± 7.6 | 16.1 ± 1.4# | N/A ± N/A |
*p ≤ 0.05 t test, aged control vs young control
#p ≤ 0.05 ANOVA, Dunnett’s post hoc vs aged control
F0: aged control = 30 months old, young control = 7 months old
n = number of breeding events (3 consecutive days of egg collection/week = 1 breeding event)
Phenotypic changes in aged fish exposed to THC
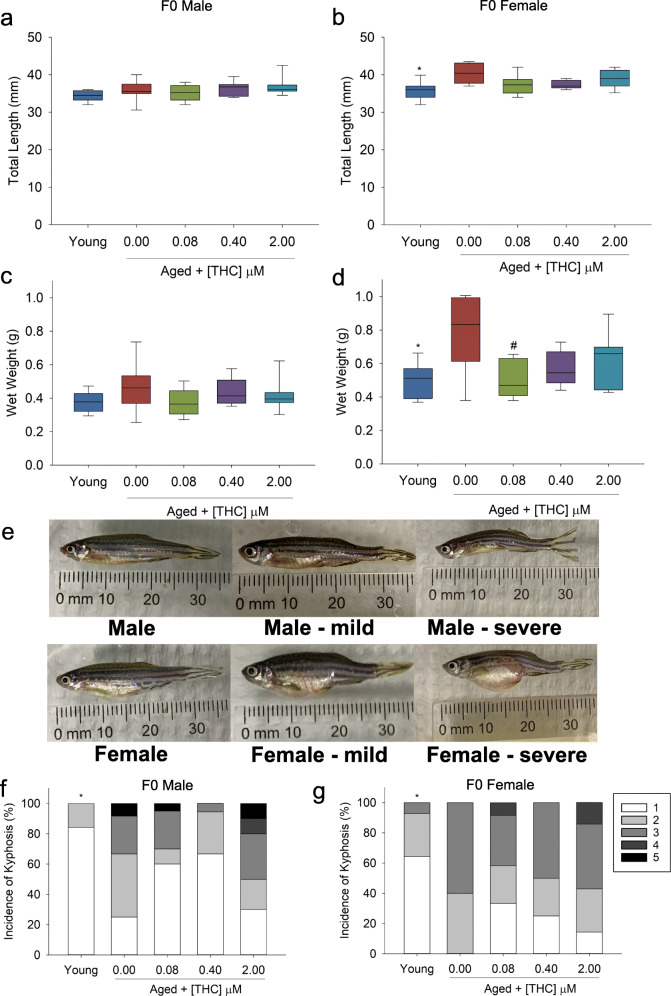
No difference in body length or mass was observed in male fish exposed to vehicle or THC during development (Fig. 3a–c). While aged female fish were significantly longer and heavier than young controls, exposure to the lowest concentration of THC (0.08 μM) throughout larval development resulted in significant reductions in mass in advanced aged females (Fig. 3b–d). However, despite the differences in size, the fish displayed similar health given that they all displayed similar healthy (k = 1) condition factor (Supplemental Figure 2A-B). Similarly, there was no difference in the presence of gross tumors in any of the THC-treated animals (Supplemental Figure 3A-B).
Fig. 3.
Weight, length, and spinal curvature (kyphosis) of adult F0 (young = 7 months old, aged = 30 months old) male (n = 8–22) & female (n = 5–12) zebrafish developmentally exposed to THC. a, b Male and female zebrafish total length (mm). c, d Male and female wet weight (g). e Male and female spinal curvature grading from healthy (1) to medium (3) to severe (5). f, g Male and female kyphosis (%). Asterisk indicates a significant difference between aged and young controls (t test, p ≤ 0.05). Number sign indicates a significant difference compared with aged controls (ANOVA, Dunnett’s posthoc, p ≤ 0.05 or Fisher’s exact test p ≤ 0.05)
The prevalence of kyphosis was then assessed. Early-life exposure to THC did not significantly affect the age-related increase in kyphosis within males or females, despite a visual trend of reduced severity with decreasing concentrations of THC (Fig. 3e–g).
Senescence and inflammation in aged fish exposed to THC
An age-related increase in the expression of inflammatory and senescence markers was quantified in the livers of the aged, control fish (Table 2). Early-life exposure to THC did not alter the increase in p16 in male or female zebrafish (Table 2). Males exposed to the lowest concentration of THC during development showed significant reductions in tnfα in advanced age (Table 2). Similarly, females exposed to low concentrations of THC also showed significant reductions in tnfα. Low levels of THC also resulted in reduced expression of il-1β in aged females (Table 2). Developmental exposure to all of the concentrations of THC in our study increased il-6 expression in males, and higher concentrations also increased pparα and pparγ expression. Conversely, the expression of pparγ was reduced in a dose-dependent manner in aged females with significant reductions observed in the 2 μM THC treatment groups (Table 2).
Table 2.
Male and female F0 (mean ± SE) THC exposed (μM) gene expression (log(2)ΔΔCt) relative to the aged controls, n = 6
| n | p16 ± SE | tnfα ± SE | il-1β ± SE | il-6 ± SE | pparα ± SE | pparγ ± SE | |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Young control | 6 | − 0.76 ± 0.61 | − 2.63 ± 0.72* | − 3.24 ± 0.30* | 1.03 ± 0.22* | 0.56 ± 0.42 | 1.46 ± 0.09* |
| Aged control | 6 | 0 ± 0.4 | 0 ± 0.78 | 0 ± 0.6 | 0 ± 0.16 | 0 ± 0.19 | 0 ± 0.23 |
| 0.08 THC | 6 | − 0.04 ± 0.04 | − 2.56 ± 0.45# | − 1.04 ± 0.26 | 0.78 ± 0.26# | 0.45 ± 0.39 | 0.48 ± 0.34 |
| 0.4 THC | 6 | 0.92 ± 0.26 | − 0.21 ± 0.50 | 0.29 ± 0.63 | 1.39 ± 0.19# | 1.98 ± 0.12# | 1.30 ± 0.34# |
| 2 THC | 6 | 1.05 ± 0.12 | 0.34 ± 0.46 | 0.8 ± 0.62 | 0.77 ± 0.28# | 0.96 ± 0.28 | 1.18 ± 0.38# |
| Females | |||||||
| Young control | 6 | − 1.13 ± 0.29 | − 4.27 ± 0.65* | − 2.84 ± 0.14* | − 0.76 ± 0.19* | 0.68 ± 0.54 | − 0.23 ± 0.38 |
| Aged control | 6 | 0 ± 0.43 | 0 ± 0.80 | 0 ± 0.56 | 0 ± 0.25 | 0 ± 0.31 | 0 ± 0.37 |
| 0.08 THC | 6 | 0.50 ± 0.24 | − 3.15 ± 0.57# | −2.14 ± 0.28# | − 0.06 ± 0.32 | 0.11 ± 0.23 | − 0.87 ± 0.20 |
| 0.4 THC | 6 | 0.31 ± 0.08 | − 2.37 ± 0.46 | − 1.98 ± 0.40# | 0.43 ± 0.35 | 0.16 ± 0.42 | − 1.00 ± 0.24 |
| 2 THC | 6 | 0.01 ± 0.26 | −1.31 ± 0.71 | −1.60 ± 0.42 | 0.65 ± 0.24 | 0.26 ± 0.32 | −1.37 ± 0.25# |
*p ≤ 0.05 t test, aged control vs young control
#p ≤ 0.05 ANOVA, Dunnett’s post hoc vs aged control
Exposure groups were normalized to 18 s, and the ΔCT values were assessed for significance by one-way ANOVA with Dunnett’s post hoc test (p ≤ 0.05), or by t test for aged compared with young (p ≤ 0.05)
Multigenerational effects of THC exposure on aging
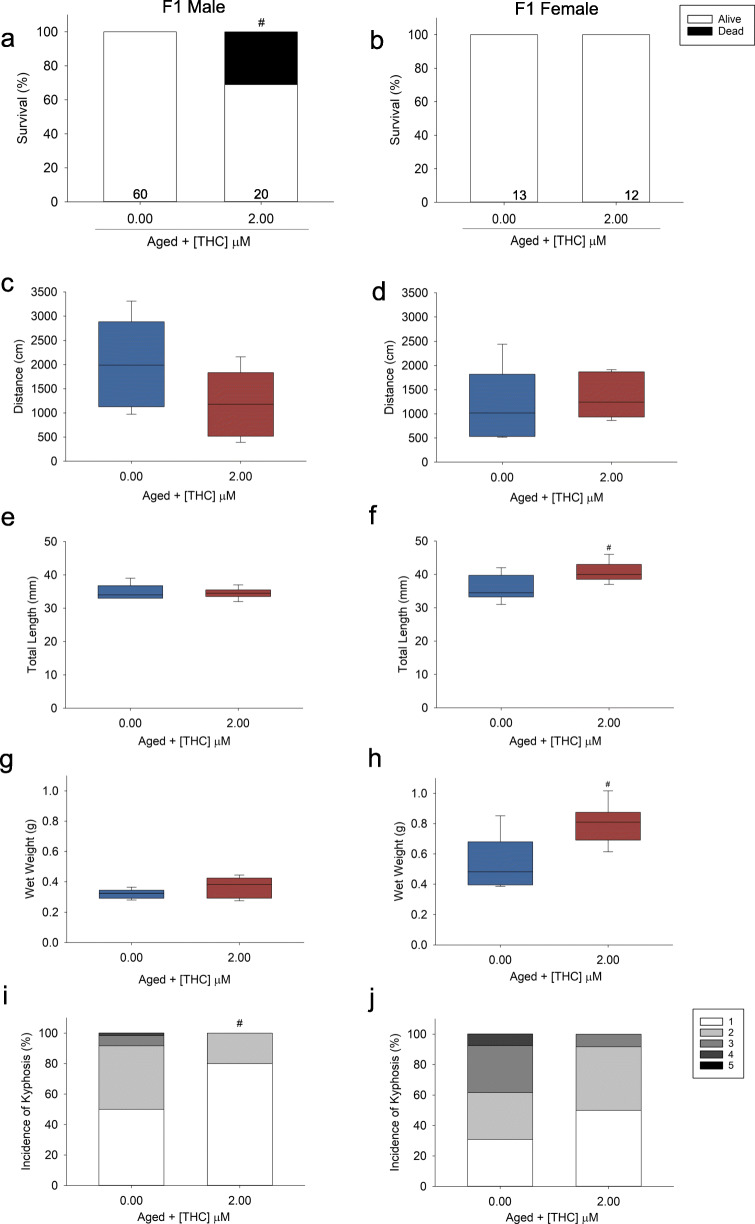
To determine whether early-life exposure to 2 μM THC influenced aging in the subsequent generation of offspring, F1 offspring were spawned at 6 months of age. There was a significant reduction (~ 30%) in male survival of the F1 THC-treated fish by 30 months of age (Fig. 4a, b). However, there were no differences in locomotor behavior (Fig. 4c, d, Supplemental Figure 4A-D). Yet, there was a significant reduction in the incidence of kyphosis of the aged F1 (parentally-exposed) male THC fish (Fig. 4i, j). The F1 2 μM parentally exposed THC fish were completely unable to reproduce unlike the aged controls (Supplemental Table 2). Despite these differences in growth and reproduction observed, there was no significant difference in gene expression between control and F1 THC offspring (Table 3).
Fig. 4.
Survival, distance traveled, length, weight, and incidence of kyphosis of male and female F1 zebrafish (parents exposed to 2 μM THC). a, b Male, n = 39–60, and female, n = 13–20, survival (%) from 12 to 30 months old. The number displayed at the base of each bar is the total number of fish per treatment enrolled into the study at 12 months old. c, d Male and female zebrafish distance traveled (cm), n = 6. e, f Weight, g, h length, and i, j spinal curvature (kyphosis) of adult F1 male (n = 11–60) and female (n = 6–19) zebrafish. Number sign indicates a significant difference compared with aged controls (ANOVA, Dunnett’s posthoc, p ≤ 0.05 or Fisher’s exact test p ≤ 0.05)
Table 3.
Male and female F1 (mean ± SE) gene expression (log(2)ΔΔCt) of fish (parents exposed to 2 μM THC) relative to the aged controls, n = 6
| n | p16 ± SE | tnfα ± SE | il-1β ± SE | il-6 ± SE | pparα ± SE | pparγ ± SE | |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Aged control | 6 | 0 ± 0.59 | 0 ± 0.9 | 0 ± 0.43 | 0 ± 0.16 | 0 ± 0.09 | 0 ± 0.19 |
| 2 μM THC | 6 | 0.4 ± 0.82 | 0.89 ± 0.85 | − 0.45 ± 0.58 | − 0.62 ± 0.62 | − 0.52 ± 0.59 | − 0.64 ± 0.52 |
| Females | |||||||
| Aged control | 6 | 0 ± 50 | 0 ± 0.57 | 0 ± 0.52 | 0 ± 0.26 | 0 ± 0.48 | 0 ± 0.18 |
| 2 μM THC | 6 | − 0.51 ± 0.55 | 0.13 ± 0.77 | − 0.86 ± 1.27 | − 0.02 ± 0.56 | − 0.92 ± 0.88 | − 1.13 ± 0.70 |
p >0.05 ANOVA, t test vs aged control
Exposure groups were normalized to 18 s and the ΔCT values were assessed for significance by t test (p ≤ 0.05)
Discussion
With the increased prevalence of cannabis use, there is an urgent need to understand the role THC plays in the Developmental Origins of Health and Disease (DOHaD). In this study, we determined if developmental exposure to THC protected or exacerbated age-related phenotypes in aged zebrafish. Similar to our concurrent submission on CBD, we observed decreased fecundity at the highest dose of THC tested. The reduction in fecundity observed at the high dose of THC in this current study supports previous studies that demonstrate maternal exposure to THC reduces the fecundity of male mice offspring 60–80 days later (Dalterio et al., 1984). While we were unable to test sperm concentration due to a shortage of male adult fish at the highest dose, it is possible that sperm concentration/quality played a role in the poor fecundity observed, as treatment with THC at the other two concentrations reduced sperm count in a dose-dependent manner. Moreover, other animal models have demonstrated the adverse effects of THC on sperm (Morgan et al., 2012). Furthermore, altered endocannabinoid signaling is linked to infertility in human sperm (Lewis et al., 2012), and the strongest evidence for the negative effects of cannabis on humans is its impacts on semen parameters such as reduced concentration (Reviewed in: Payne et al., 2019).
Sex-specific effects in aging are frequently reported (Austad, 2019), and our current study supports previous research on sex-specific effects of THC (reviewed in: Brents, 2016). THC alters the hypothalamic-pituitary-ovarian (HPO) axis functionality, while hormones produced by the HPO axis impact the physiological and behavioral effects of THC (Brents, 2016). Because estradiol/progesterone play a larger role in modulating THCs effects, female rats display greater tolerance to THC than males (Marusich et al., 2015; Wakley et al., 2014). Female tolerance has also been demonstrated for other cannabinoids such as WIN55,212 in rats, with male rats exhibiting behavioral and neuronal deficits and females being unaffected (Bara et al., 2018). Together, our results suggest that developmental exposure to THC can cause persistent sex-specific alterations to the reproductive system, with high concentrations of THC causing negative outcomes in male fish. However, our results of increased egg production in this current study are not congruent with our previous findings that saw fewer eggs produced by 0.08 and 0.4 μM THC-treated fish when bred at 6 months of age (Carty et al., 2019). Collectively, the effect of THC on female egg production may be dependent on the stage of life in which it is assessed—decreased egg production measured early and increased egg production later.
Because the negative effects of THC on sperm is not CB1-mediated (Morgan et al., 2012), the effect of THC on reproduction is likely mediated through another receptor/mechanism. THC treatment significantly reduces ATP levels in sperm (Morgan et al., 2012). Thus, the reproductive effects could be a result of altered metabolism or mitochondrial function. In this study, we observed significant sex-dependent changes in expression of pparα and pparγ, with increased expression in males, and decreased expression of pparγ in females. This reduction in pparγ was also observed when we exposed zebrafish to CBD (concurrent submission). PPARγ regulates adipogenesis (Berger and Moller, 2002; Bouaboula et al., 2005; Gerhold et al., 2002), and lifelong alteration of energy metabolism in cannabinoid-exposed females could have contributed to the reduced weight observed in both CBD- and THC-treated fish. Because sexual maturation is size dependent in zebrafish (Parichy et al., 2009), it is possible the CBD and THC exposed female fish simply matured later than the aged controls, resulting in lower egg production at a younger age and higher egg production later. The heavier aged female control fish could be indicative of age-related obesity, which can contribute to chronic inflammation (Jura and Kozak, 2016).
Increased lifespan was generally accompanied by reduced expression of tnfα and il-1β in both males and females, which is consistent with other animal models that show increased pro-inflammatory cytokines in advanced age (Bollaerts et al., 2017; Royce et al., 2019; Van houcke et al., 2015). However, the effect of THC on the expression of il-6 was different than tnfα and il-1β. Our results suggest IL-6 may play an important sex-dependent regulatory role as its expression was significantly decreased in aged male fish, and this decrease was rescued in THC-treated males. While there are few studies in zebrafish studying IL-6 in a regulatory role, it is involved in the regulation of hematopoiesis (Pazhakh and Lieschke, 2018). The anti-inflammatory properties of THC have been well-studied (Hegde et al., 2008; Klein et al., 1998; Nagarkatti et al., 2009) and are mediated by CB2 and in some cases CB1. Our results in this current study as well as our CBD exposure study (concurrent submission) indicate that developmental exposure to cannabinoids can alter the expression of pro-inflammatory cytokines in aged fish, suggesting a possible reduction in inflammaging throughout the lifespan. Importantly, this reduction of chronic inflammation could be responsible for the increased longevity observed.
The concentration-dependent effects of THC on tnfα and il-1β gene expression were biphasic/hormetic, wherein the most downregulation was seen at the lowest dose tested. Low doses of THC protect mice from neuroinflammation-induced damage (Fishbein-Kaminietsky et al., 2014). Interestingly, the protective effect of THC was blocked with a CB1 and PPARγ but not CB2 antagonist (Fishbein-Kaminietsky et al., 2014). While exposure to low doses can cause long-term neuroprotective effects observable 7 weeks post exposure (Fishbein et al., 2012), in our study we observed persistent effects 30 months following developmental exposure. Thus exposure during early developmental periods can cause persistent alterations to gene expression.
While adult THC-exposed fish weighed less in a biphasic manner, we observed increased size in the F1 parentally exposed offspring as THC concentration decreased (exactly the opposite of what we saw in the adults). This effect along with the change in the eye diameter offer evidence that exposure to THC during development can cause developmental alteration across generations. We unfortunately only kept the highest concentration of THC-exposed fish to raise into adulthood for the F1 generation due to space constraints. Future studies should assess the multigenerational effects of lower doses to THC, as there were many effects observed in the F0. The effect of parental exposure to the high dose of THC continued to have adverse outcomes in the F1 generation as they grew into adulthood with the fish exhibiting lower survival and they were completely unable to reproduce when bred at 30 months old. However, there was no significant alteration of gene expression in the F1 parentally exposed progeny. Overall, the highest concentration of THC tested displayed toxicity to their F1 progeny measured through reduced survival and complete reproductive failure when aged.
Along with our concurrent CBD study, the data presented here indicate developmental exposure to THC and CBD can cause significant alterations in the health and function in advanced age. While some of the effects of THC and CBD were similar, there were substantial differences which are likely brought about by the varying mechanisms of action of each compound. Additionally, we used a different concentration range for THC (0.08, 0.4, and 2 μM) and CBD (0.02, 0.1, and 0.5 μM) because of different potencies in acute developmental toxicity (Carty et al., 2018). By far, the most contrasting result was that CBD generally acted in a protective, concentration-dependent manner being most effective at 0.5 μM, while THC’s effects were biphasic (most effective at 0.08 μM) for many of the endpoints observed including survival, weight and length, kyphosis, and the expression of genes such as tnfα and il-1β. However, exposure to CBD and THC displayed concentration-dependent reductions in fecundity at the highest concentrations exposed (0.5 μM or 2 μM, respectively), as well as similar increased aged female egg production at lower concentrations. Exposure to either cannabinoid caused a concentration-dependent reduction of pparγ expression in female fish, but in male fish THC increased expression, whereas CBD did not. Taken together, cannabinoid exposure during early development can cause significant long-term effects on reproduction, growth, and survival.
In this current study, high dose exposure of THC resulted in reproductive toxicity, significantly reducing the survival and reproduction of F1 progeny well into adulthood. Low dose exposure to THC reduced the expression of genes related to senescence and inflammation and increased survival. Due to the biphasic nature of THC, future research considering long-term and multigenerational impacts of low doses of THC merit further study. Future work in additional animal models exposed to cannabinoids at various ages is now necessary to determine whether low doses of THC and/or non-psychoactive cannabinoids have the potential to modulate the aging process.
Electronic supplementary material
(DOCX 418 kb)
Acknowledgments
The authors would like to thank Dennis R. Carty for conducting the initial exposure, as well as Kennedy E. Dickson, Mary-Beth Gillespie, James H. Gledhill, Haley Watts, and Bailey Westling for their help during sampling.
Author contributions
KW, NA, and ZP conceived and designed the experiments; NA and ZP analyzed the data; AF, AW, CT, KL, NA, and ZP performed the experiments; KW, NA and ZP prepared the manuscript.
Funding information
This work was supported by the National Institute on Drug Abuse R21DA044473-01 grant awarded to KW and P30GM122733 to NMA and KW.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E. Key substance use and mental health indicators results from the 2016 national survey in the United States: on drug use and health; 2017. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm.
- Assaf F, Fishbein M, Gafni M, Keren O, Sarne Y. Pre- and post-conditioning treatment with an ultra-low dose of Δ9-tetrahydrocannabinol (THC) protects against pentylenetetrazole (PTZ)-induced cognitive damage. Behav Brain Res. 2011;220:194–201. doi: 10.1016/j.bbr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Austad SN. Sex differences in health and aging: a dialog between the brain and gonad? GeroScience. 2019;41:267–273. doi: 10.1007/s11357-019-00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, Gard CC, Wright LL, LaGasse L, Higgins R. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- Bara A, Manduca A, Bernabeu A, Borsoi M, Serviado M, Lassalle O, Murphy M, Wager-Miller J, Mackie K, Pelissier-Alicot AL, Trezza V, Manzoni OJ. Sex-dependent effects of in utero cannabinoid exposure on cortical function. Elife. 2018;7:1–31. doi: 10.7554/eLife.36234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, Dvir-Ginzberg M, Rácz I, Ulas T, Imbeault S, Bab I, Schultze JL, Zimmer A. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23:782–787. doi: 10.1038/nm.4311. [DOI] [PubMed] [Google Scholar]
- Bollaerts I, Van Houcke J, Andries L, De Groef L, Moons L. Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediat Inflamm. 2017;2017:1–14. doi: 10.1155/2017/9478542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARγ transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Brents LK. Marijuana, the endocannabinoid system and the female reproductive system. Yale J Biol Med. 2016;89:175–191. [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? Npj Aging Mech Dis. 2017;3:1–8. doi: 10.1038/s41514-017-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Li Y, Liu H, Bai G, Mayl J, Lin X, Sutherland K, Nabar N, Cai J. The potential therapeutic effects of THC on Alzheimer’s disease. J Alzheimers Dis. 2014;42:973–984. doi: 10.3233/JAD-140093. [DOI] [PubMed] [Google Scholar]
- Carty DR, Thornton C, Gledhill JH, Willett KL. Developmental effects of cannabidiol and Δ9-tetrahydrocannabinol in zebrafish. Toxicol Sci. 2018;162:137–145. doi: 10.1093/toxsci/kfx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty DR, Miller ZS, Thornton C, Pandelides Z, Kutchma ML, Willett KL. Multigenerational consequences of early-life cannabinoid exposure in zebrafish. Toxicol Appl Pharmacol. 2019;364:133–143. doi: 10.1016/j.taap.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J, Fang X, Thornton C, Mei W, Barbazuk WB, Duke M, Scheffler BE, Willett KL. Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo[a]pyrene exposure. Comp Biochem Physiol C Toxicol Pharmacol. 2014;163:37–46. doi: 10.1016/j.cbpc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalterio SL, DeRooij DG. Maternal cannabinoid exposure effects on spermatogenesis in male offspring. Int J Androl. 1986;9:250–258. doi: 10.1111/j.1365-2605.1986.tb00888.x. [DOI] [PubMed] [Google Scholar]
- Dalterio S, Steger R, Mayfield D, Bartke A. Early cannabinoid exposure influences neuroendocrine and reproductive functions in male mice: I prenatal exposure. Pharmacol Biochem Behav. 1984;20:107–113. doi: 10.1016/0091-3057(84)90110-2. [DOI] [PubMed] [Google Scholar]
- Desai A, Mark K, Terplan M. Marijuana use and pregnancy prevalence, associated behaviors, and birth outcomes. Obstet Gynecol. 2014;123:46S–111. doi: 10.1007/s00737-015-0529-9. [DOI] [Google Scholar]
- El-Mohandes A, Herman AA, El-Khorazaty MN, Katta PS, White D, Grylack L. Prenatal care reduces the impact of illicit drug use on perinatal outcomes. J Perinatol. 2003;23:354–360. doi: 10.1038/sj.jp.7210933. [DOI] [PubMed] [Google Scholar]
- Feltes BC, de Faria Poloni J, Bonatto D. Development and aging: two opposite but complementary phenomena. Interdiscip Top Gerontol. 2015;40:74–84. doi: 10.1159/000364932. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG Int J Obstet Gynaecol. 2002;109:21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Gómez-Ruiz M, García C, Hernández M, Ramos JA. Modeling neurodegenerative disorders for developing cannabinoid-based neuroprotective therapies. Methods Enzymol. 2017;593:175–198. doi: 10.1016/bs.mie.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Zhang C, Boschen KE, Boa-Amponsem O, Mendoza-Romero HN, Tarpley M, Chdid L, Mukhopadhyay S, Cole GJ, Williams KP, Parnell SE. Cannabinoids exacerbate alcohol teratogenesis by a CB1-hedgehog interaction. Sci Rep. 2019;9:1–16. doi: 10.1038/s41598-019-52336-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein M, Gov S, Assaf F, Gafni M, Keren O, Sarne Y. Long-term behavioral and biochemical effects of an ultra-low dose of Δ9-tetrahydrocannabinol (THC): neuroprotection and ERK signaling. Exp Brain Res. 2012;221:437–448. doi: 10.1007/s00221-012-3186-5. [DOI] [PubMed] [Google Scholar]
- Fishbein-Kaminietsky M, Gafni M, Sarne Y. Ultralow doses of cannabinoid drugs protect the mouse brain from inflammation-induced cognitive damage. J Neurosci Res. 2014;92:1669–1677. doi: 10.1002/jnr.23452. [DOI] [PubMed] [Google Scholar]
- Gerhold DL, Franklin L, Jiang G, Zhihua LI, Jian XU, Meiqing LU, Sachs JR, Bagchi A, Fridman A, Holder DJ, Doebber TW, Berger J, Elbrecht A, Moller DE, Zhang BB. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-γ agonists. Endocrinology. 2002;143:2106–2118. doi: 10.1210/endo.143.6.8842. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Extending injury- and disease-resistant CNS phenotypes by repetitive epigenetic conditioning. Front Neurol. 2015;6:1–7. doi: 10.3389/fneur.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen AC, Schug TT, Collman G, Heindel JJ. Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis. 2015;6:55–64. doi: 10.1017/S2040174414000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, Najman JM. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215–219. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges EL, Ashpole NM. Aging circadian rhythms and cannabinoids. Neurobiol Aging. 2019;79:110–118. doi: 10.1016/j.neurobiolaging.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol Teratol. 2005;27:221–229. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Omaha). 2016. 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed]
- Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–115. doi: 10.1016/S0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. 2015;213:201.e1–201.e10. doi: 10.1016/j.ajog.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SEM, Rapino C, Di Tommaso M, Pucci M, Battista N, Paro R, Simon L, Lutton D, Maccarrone M. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Craft RM, Lefever TW, Wiley JL. The impact of gonadal hormones on cannabinoid dependence. Exp Clin Psychopharmacol. 2015;23:206–216. doi: 10.1037/pha0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ, Muller CH, Murataeva NA, Davis BJ, Mackie K. Δ 9-tetrahydrocannabinol (Δ 9-THC) attenuates mouse sperm motility and male fecundity. Br J Pharmacol. 2012;165:2575–2583. doi: 10.1111/j.1476-5381.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandelides Z, Thornton C, Faruque AS, Whitehead AP, Willett KL, Ashpole NM. Developmental exposure to cannabidiol (CBD) alters longevity and health span of zebrafish (Danio rerio). GeroScience. 2020. 10.1007/s11357-020-00182-4. [DOI] [PMC free article] [PubMed]
- Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of post-embryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn. 2009;238:9–12. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne KS, Mazur DJ, Hotaling JM, Pastuszak AW. Cannabis and male fertility: a systematic review. J Urol. 2019;202:674–681. doi: 10.1097/JU.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazhakh V, Lieschke GJ. Hematopoietic growth factors: the scenario in zebrafish. Growth Factors. 2018;36:196–212. doi: 10.1080/08977194.2019.1567506. [DOI] [PubMed] [Google Scholar]
- Ramírez BG, Blázquez C, Gómez Del Pulgar T, Guzmán M, De Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, B., Jennings, M., 2011. Guidance on the housing and care of zebrafish (Danio rerio), Research Animals Department, Science Group, RSPCA 10.1111/evo.12990.
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J. 2006;20. 10.1096/fj.06-5864fje. [DOI] [PubMed]
- Royce GH, Brown-Borg HM, Deepa SS. The potential role of necroptosis in inflammaging and aging. GeroScience. 2019;41:795–811. doi: 10.1007/s11357-019-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to imageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder MMHJ, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N. Characteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based study. Drug Alcohol Depend. 2010;109:243–247. doi: 10.1016/j.drugalcdep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Van Houcke J, De Groef L, Dekeyster E, Moons L. The zebrafish as a gerontology model in nervous system aging, disease, and repair. Ageing Res Rev. 2015;24:358–368. doi: 10.1016/j.arr.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH. Differential effects of Δ9-THC on spatial reference and working memory in mice. Psychopharmacology. 2001;157:142–150. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA - J Am Med Assoc. 2019;322:167–169. doi: 10.1001/jama.2019.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2014;143:22–28. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer’s disease. Rev Neurosci. 2005;16:325–337. doi: 10.1515/REVNEURO.2005.16.4.325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 418 kb)