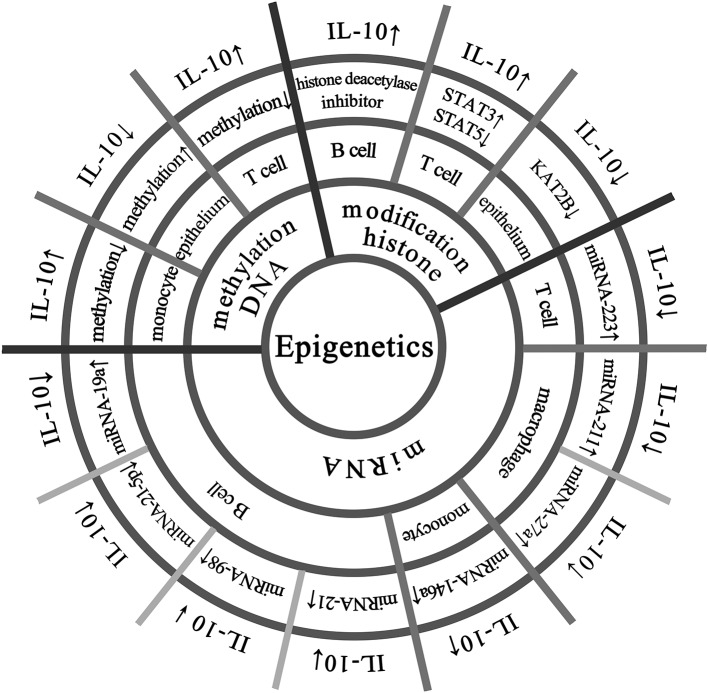
Figure 1.
Epigenetic modifications that regulate IL-10 expression in multiple cells. The IL-10 promoter has transcriptional activity and hypomethylation in human peripheral blood mononuclear cells, while the IL-10 promoter is silenced in epithelial cells due to hypermethylation. There is an enhancer in the IL-10 gene intron in T cells, and its hypomethylation promotes IL-10 expression. B cells treated with histone deacetylase inhibitor and 5-aza can increase the expression of IL-10. In T cells, increased activation of STAT3 can lead to enhanced recruitment of regulatory regions and competitive replacement of STAT5, promoting IL-10 expression. In normal colonic epithelial cell line (NCM460), laccase acid inhibits KAT2B and reduces the transcriptional activity of KAT2B and H4K5ac on the IL-10 promoter, thereby significantly down-regulating the expression of IL-10. Overexpression of miRNA-146a in peripheral blood mononuclear cells increases IL-10 expression. Up-regulation of miRNA-19a in B cells reduces IL-10 expression, and the same up-regulation of miRNA-98 can also suppress IL-10 expression, and insufficient expression of miRNA-21-5p is one of the reasons for the decrease in IL-10 expression in B cells. Up-regulation of miRNA-21 inhibits the differentiation of IL-10 + Breg cells and promotes the expression of miRNA-223 in autoimmune T cells, resulting in reduced IL-10 production. In macrophages, the relative expression of miRNA-211 is abnormally upregulated, accompanied by a decrease in secreted IL-10. miRNA−27a can enhance the antibacterial activity of macrophages and inhibit the expression of IL-10.