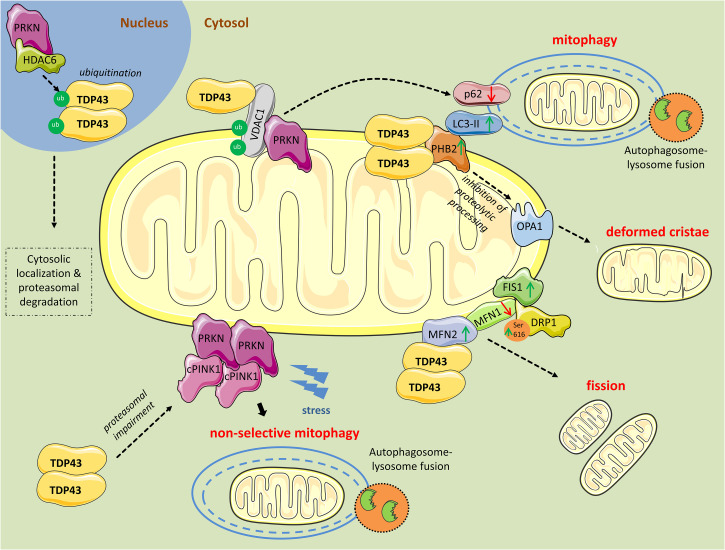
FIGURE 1.
Mitochondrial perturbations induced by TDP43. PRKN in complex with HDAC6, ubiquitinates nuclear TDP43 promoting its cytoplasmic localization and proteasomal degradation. However, as revealed from research in aging or neurodegenerative diseases, TDP43 often persists in the cytosol and forms aggregates. Excess cytosolic TDP43 interacts with VDAC1, located in the outer mitochondrial membrane, but it is still unclear if interferes with its functions. Polyubiquitination of VDAC1 by PRKN is essential for driving mitophagy. Moreover, cytosolic TDP43, translocated to the outer mitochondrial membrane, directly interacts with PHB2 and, in parallel, increases its protein levels. PHB2 is known to interact with LC3-II to induce mitophagy. PHB2 is also involved in mitochondrial membranes fusion by stabilizing indirectly the long forms of OPA1. Additionally, TDP43 directly interacts with MFN2, a mitochondrial membrane protein regulating mitochondrial fusion, and possibly stabilizes its expression. Concurrently, TDP43 leads to reduced levels of another fusion protein, MFN1, and increases levels of FIS1 and DRP1 phosphorylated at Ser616, proteins promoting mitochondrial fission. Finally, TDP43 downregulates PRKN mRNA and protein levels, and impairs the proteasome, leading to the accumulation of cleaved PINK1 (cPINK1) in the cytosol. During stress conditions cPINK1 aggregates recruit PRKN to the mitochondria launching mitophagy in otherwise healthy mitochondria (non-selective mitophagy).