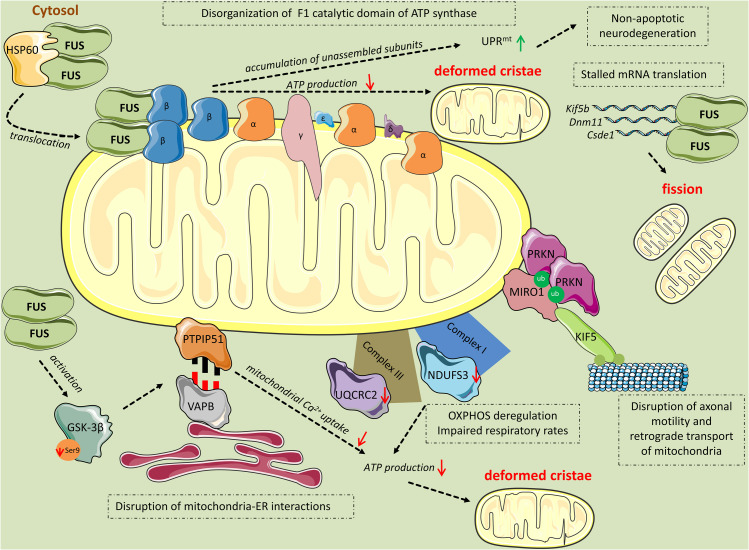
FIGURE 2.
Mitochondrial perturbations induced by FUS. HSP60 mediates FUS translocation to the outer mitochondrial membrane. Mitochondrial-localized FUS binds to the β subunit of the F1 catalytic domain of ATP synthase (Complex V). The binding leads to disassembly of the F1 domain and accumulation of unassembled ATP synthase subunits, including ATP5B, which activates the UPRmt response leading to non-apoptotic cell death. Additionally, disruption of the F1 domain of the ATP synthase complex results in impaired ATP production and thereafter, deformed cristae. FUS induces mitochondrial perturbations in several other manners while being in excess in the cytoplasm. Mutant FUS binds to mature mRNAs coding for important mitochondrial proteins including Kif5b, Dnm1l, and Csde1, inhibiting their translation. This inhibition progressively leads to mitochondrial fission. Excess FUS drives the accumulation of PINK1 and PRKN proteins. As a consequence, RHOT1, also known as Miro1, a component of the primary motor/adaptor complex that anchors kinesin to the mitochondrial surface and a direct target of PRKN, is ubiquitinated leading to disruption in axonal motility and retrograde transport of mitochondria. Additionally, FUS has an impact on the OXPHOS process by deregulating the expression of the subunits NDUFS3 and UQCRC2 of Complexes I and III, respectively. OXPHOS deregulation leads to respiratory impairment and subsequent ATP production deterioration and deformed cristae. Finally, FUS decreases the levels of ser9 phosphorylation in GSK-3β, leading to increased GSK-3β activity. Activated GSK-3β deregulates the interaction of mitochondrial tethered membrane protein PTPIP51 and the inner protein of the ER, VAPB, disrupting mitochondria-ER associations. The ER-mitochondria disruption decreased Ca2+ uptake by mitochondria following release from ER stores, resulting in reduced ATP production and deformed mitochondria.