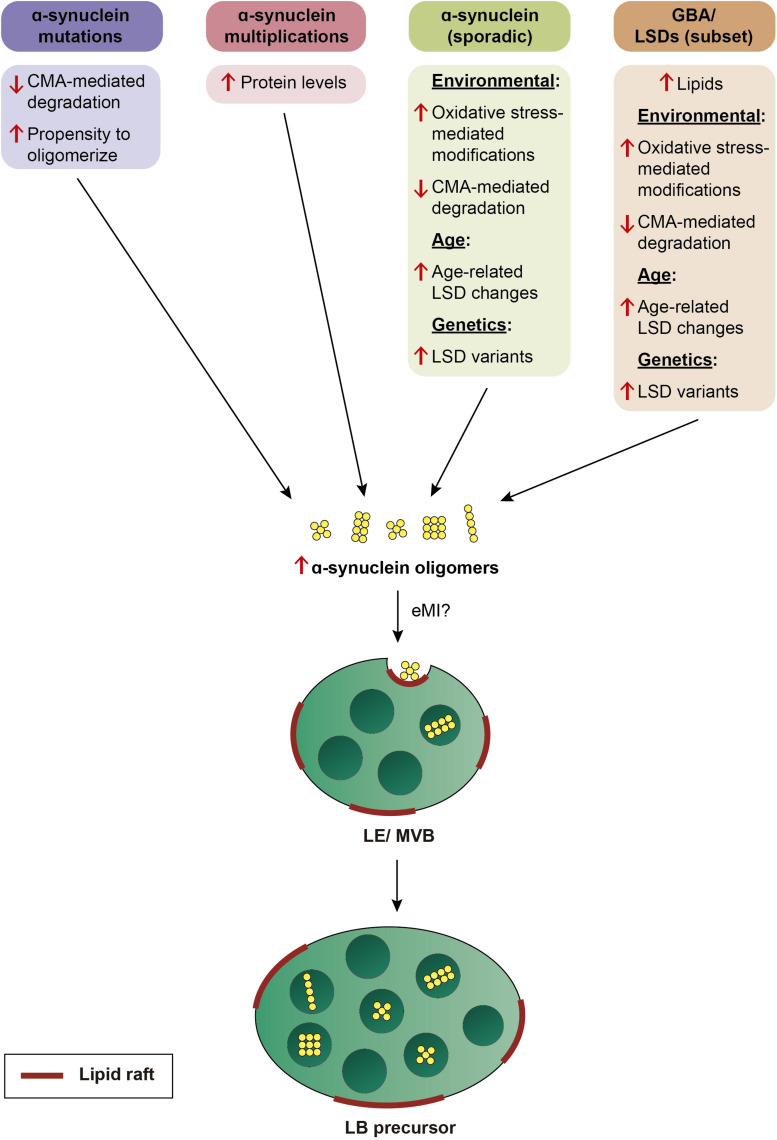
FIGURE 3.
Model by which various genetic alterations cause α-synuclein oligomerization and eventual LB pathology. Mutations in α-synuclein cause oligomerization due to an enhanced propensity of the mutant protein to oligomerize, and/or due to its impaired turnover by CMA. Multiplication of the α-synuclein locus causes oligomerization over time due to an increase in protein levels. In sporadic PD, α-synuclein oligomerization is triggered either by environmental factors, resulting in oxidative stress-mediated posttranslational modifications which interfere with its turnover by CMA, or by age-related or genetic alterations in LSD enzyme activity which result in changes in the membrane lipid composition. In a subset of LSDs including mutations in GBA, oligomerization of α-synuclein is triggered by altered lipid composition in conjunction with additional environmental, age-dependent, or genetic alterations. In all cases, α-synuclein oligomers may be inefficiently cleared by eMI, causing a toxic buildup of cytosolic oligomers over time. The eMI-mediated internalization of α-synuclein oligomers at the LE/MVB may cause the formation of LB precursors over time.