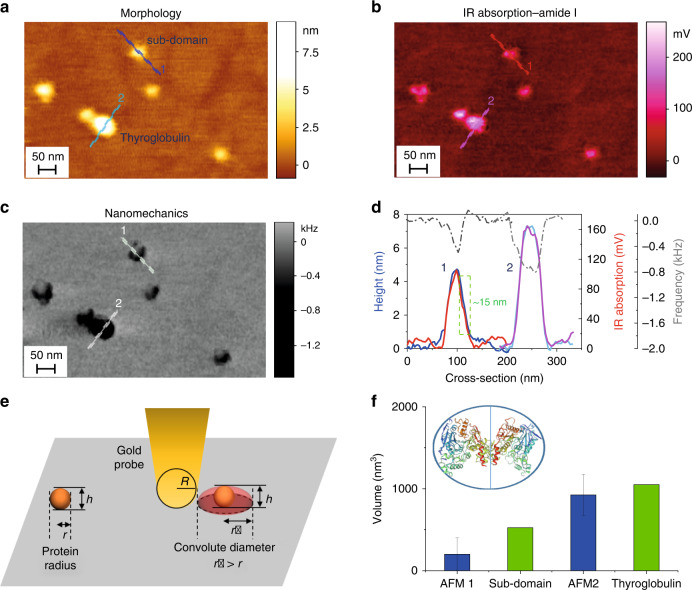
Fig. 2. Simultaneous morphological, chemical and mechanical imaging by IR absorption of single protein molecules.
a 3D morphology map of thyroglobulin molecules. b IR absorption image at 1655 cm−1 (amide band I) and c contact resonance frequency map at 1655 cm−1 (laser power: 0.24 mW; pulse: 100 ns). d Cross-sectional correlation of morphology, IR absorption and nanomechanical properties for the protein species in the map. e An overestimate of the lateral radius r and of the volume of the single proteins in the AFM-IR map is caused by the finite geometrical shape and radius of the tip31,32. f Inset of the graph is the structure of a thyroglobulin molecule, which is composed by two identical subdomains29. We demonstrate single protein sensitivity by comparing the known volumes of a thyroglobulin molecule (blue) and its subdomain (green)30, with the deconvoluted volume of each single protein species measured on the surface by AFM (blue, the error on the deconvoluted volume is calculated by varying the radius of the tip between R ~30 and 40 nm) (Supplementary Fig. 6)31. The volume of a single protein measured by AFM (blue) is in excellent agreement with the values of the volume of a single subdomain and thyroglobulin. Source data are provided as a Source Data file.