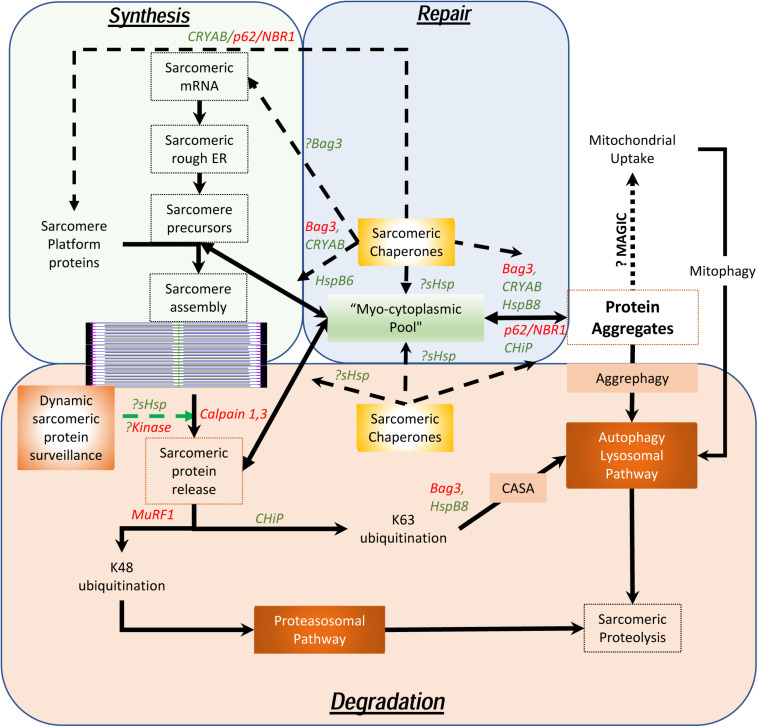
FIGURE 2.
Proposed model of the cardiac sarcostat. In this model, mRNA and proteins are located in stoichiometric excess, in vicinity of the sarcomere, as a myocytoplasmic pool. As most sarcomeric proteins are highly insoluble and aggregate prone, concerted action of a variety of sarcomeric chaperones (in green letters) and chaperone associated sarcomeric proteins (in red letters) is required to ensure normal sarcomere structure and function. These chaperones also appear to be integral to sarcomere assembly as well as repair and maintenance of sarcomeric proteins, in situ. When the damaged proteins are detected (often due to exposure of kinase sites) by the “sarcostat damage sensing mechanism,” the proteins are removed from the sarcomere (via sarcomeric calpains) and returned to the myocytoplasmic reserve pool. When sarcomeric proteins acquire unfolded states due to nascent status, or are misfolded as a result of synthetic errors or due to damage, sarcostat chaperones assist in their refolding and in enabling dynamic equilibrium between the sarcomere-associated and the reserve pools. The proteins that are beyond the capacity of the refolding mechanism or require to be stored in the anticipation of continued stress, undergo aggregation under the action of Bag3, CryAB, and p62. As a result of liquid-liquid phase transition, these proteins and their cognate chaperones remain outside the osmotic load of the cell, stacked in a compact arrangement due to their intrinsic disordered domains (IDRs). These aggregates can be a source to release protein back in to the myocytoplasmic pool and, thus, the sarcomere. Normal lysosomal function and, possibly mitochondrial aggregate uptake (via a pathway termed as “MAGIC,” see text), result in continuous flux of these aggregates. In addition, normal proteolysis of isolated proteins occurs via the ubiquitin proteasome system and the autophagy-lysosomal pathway. During states when the ubiquitin proteasome pathway is inhibited, proteins are diverted into storage as protein aggregates or for degradation via the autophagy-lysosomal pathway. As many disease states also result in lysosomal dysfunction, the accumulation of the protein aggregates accompanies failure of the sarcostat. In these states, upregulation of the sarcostat protein degradation machinery (both UPS and ALP), results in improved sarcomeric protein quality, while concurrently increasing aggregate removal.