Abstract
Immune checkpoint inhibitors (ICIs) improve significantly outcome of patients with advanced renal cancer. Although immune-related adverse events involve frequently skin, digestive tract, lung, liver and endocrine organs, haematological toxicities are rare. We describe the case of a patient with metastatic renal cancer who was treated with nivolumab. Eight courses of nivolumab were administered without any toxicity; brain metastases were then diagnosed and treated with stereotactic radiotherapy. As the extra-cranial disease was stable, the ninth course of nivolumab was administered 5 days after the end of radiotherapy. One week later, he presented with rectal and nasal bleeding in a context of severe thrombocytopenia (1000/mm3). High dose of steroids and intravenous immunoglobulin reversed slowly the thrombocytopenia. This case highlights the possibility of life-threatening thrombocytopenia with ICIs. Interestingly, the close time relation with radiotherapy highlights a potential interaction, warranting a close follow-up of patients in this situation.
Keywords: cancer intervention, oncology, urological cancer, immunology
Background
Immune checkpoint inhibitors (ICIs) improve the outcome of patients with metastatic renal cancer. The programmed death-1 (PD-1) receptor is expressed on the surface of activated T-cells; the binding of PD-1 to its ligand PD-L1, which is expressed on the surface of antigen-presenting cells (APC) such as macrophages and dendritic cells, transmits an inhibitory signal into T-cell, reducing its activity, its proliferation and the cytokine production. Multiple cancer cells highly express PD-L1 on their surface, allowing mechanism to escape immune system. By inhibiting the PD-1/PD-L1 axis, the monoclonal antibody anti-PD1 nivolumab enables the activation of T-cells, restoring lymphocyte ability to efficiently detect and attack tumour cells.1 Nivolumab is currently approved in metastatic renal cancer after failure of tyrosine kinase receptor (TKI).2 Although better tolerated and with less adverse events (AEs) than tyrosine kinase inhibitor, ICIs have been reported to induce severe and potentially life-threatening immune-related AEs (irAEs). The most frequent are rash, colitis, pneumonitis, hepatitis and endocrinopathy. Life-threatening irAEs are rarely described but are probably underestimated.3–5 These AEs can appear lately in the treatment course and can also reverse very slowly despite high-dose corticosteroids. Moreover, interactions with other treatment modalities could probably exist and should be reported. We report the case of a patient who presented a severe thrombocytopenia that occurred during nivolumab course in a close time relation with stereotactic radiotherapy.
Case presentation
In September 2018, a 56-year-old patient presented with haematuria and abdominal pain. His medical history was not relevant. A 10 cm left kidney mass was diagnosed with multiple centimetric pulmonary nodes. A radical nephrectomy was performed and confirmed clear-cell renal cell carcinoma (ccRCC). A cerebral MRI was at this time negative. In December 2018, sunitinib was started due to increase of size of pulmonary metastases. Sunitinib was well tolerated; AEs were mainly grade 2 fatigue and grade 2 mucositis. In April 2019, due to progression of pulmonary metastases (figure 1), nivolumab was started (240 mg/body every 2 weeks). Six courses were administered with no reported AE; particularly, blood test was performed every 2 weeks without any haematological abnormality. In July 2019, after the sixth administration, the thoraco-abdominal CT showed disease stability according to RECIST version 1.1 criteria and nivolumab was thus continued for two further courses. In August 2019, in a context of headache, a cerebral MRI showed two brain metastases (diameter of 25 mm and 20 mm) with low degree of oedema (figure 2). Oral methylprednisone (32 mg daily) rapidly improved the symptoms and stereotaxic ablative radiotherapy (SABR) was performed (one single fraction of 20 Gy on each metastasis) 10 days later. Methylprednisone was stopped 5 days after radiotherapy and the ninth course of nivolumab was administered 7 days after radiotherapy; it is important to note that the blood test performed the day before nivolumab administration was completely normal. Two weeks later, he presented in emergency for rectal and nasal bleeding (day 1). The blood control showed grade 4 thrombocytopenia (6000/mm³).
Figure 1.
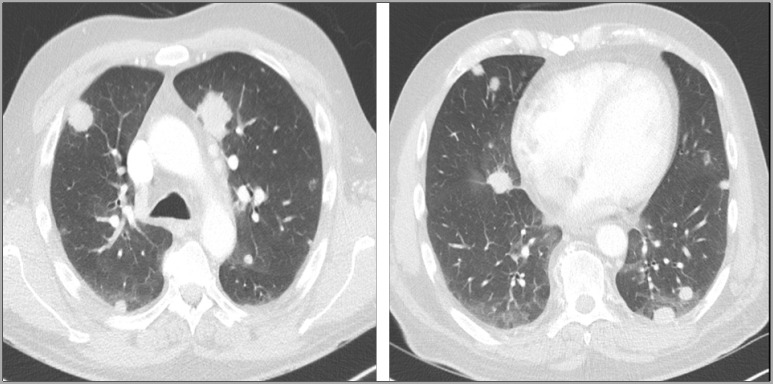
Lung metastases in progression on sunitinib.
Figure 2.
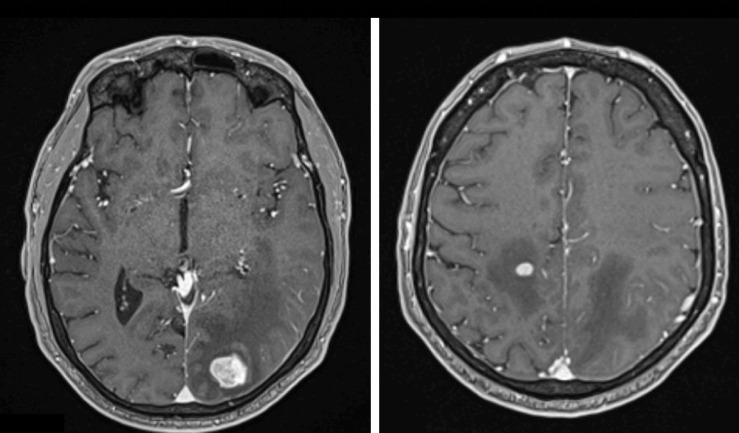
Brain metastases treated with stereotactic ablative radiotherapy.
Investigations
Haemoglobin was within normal range as well as leucocytes and reticulocytes. Renal function was stable compared with previous values (creatinine 1.2 mg/dL and urea 45 mg/dL). No shizocytes were detected and haptoglobin was within normal range. Lactate dehydrogenase (LDH), bilirubin and hepatic values were normal. Thyroid test (T4 and TSH) was normal. Auto-immune antibodies (antinuclear factor) were negative. The bone marrow aspiration excluded central aetiology. Cytomegalovirus, Epstein-Barr virus and hepatitis B and C serologies were negative.
Treatment
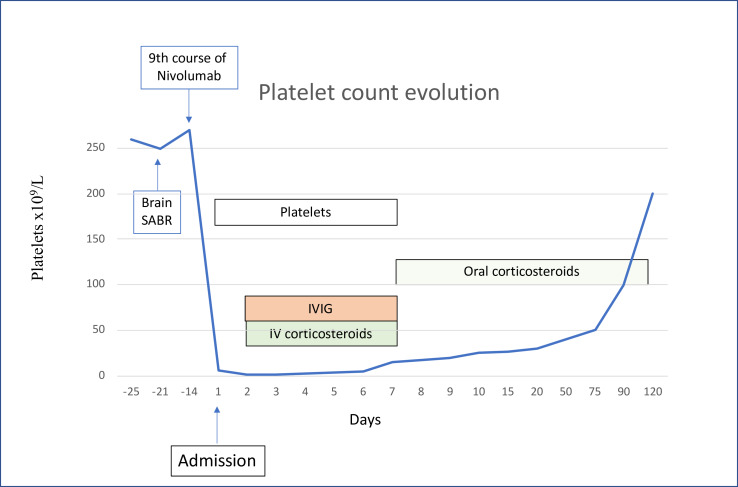
Despite multiple transfusions, platelets continued to decrease, reaching 1000/mm³ on day 2 with increasing bleeding episodes; intravenous corticosteroids (methylprednisone 2 mg/kg daily) were then started in association with intravenous immunoglobulin (IVIG; privigen) 1 mg/kg/day. At day 7, platelet count reached 15×109/L; as bleeding was significantly decreased, platelet transfusions and privigen were stopped and methylprednisone was continued orally (64 mg daily). Platelet count reached 25 000/mm3 at day 10, 30 000/mm3 at day 20, 40 000 at day 50 and 50 000 at day 75; methylprednisone was at this time decreased to 32 mg daily. At day 90, platelets reached 100 000/mm³ and methylprednisone was progressively tapered and stopped at day 120 (figure 3). Nivolumab was never reintroduced.
Figure 3.
Platelet count evolution. IVIG, intravenous immunoglobulin; SABR, symptoms and stereotaxic ablative radiotherapy.
Outcome and follow-up
In January 2020, thoraco-abdominal CT showed progressive disease with new lung metastases and cabozantinib was started (40 mg daily). Eight weeks later, tolerance remains correct without any sign of haematological toxicity and thoraco-abdominal CT showed partial response.
Twelve weeks after cabozantinib introduction, our patient is still alive without any haematological toxicity.
Discussion
We described an interesting case of thrombocytopenia occurring in a patient with renal cancer treated with nivolumab. This first hypothesis is an immune-related toxicity induced by nivolumab. Haematological-irAEs are rare events; in a recent review of large clinical trials, the frequency was estimated at 3.6% for all Common Terminology Criteria for Adverse Events (CTCAE) grades and 0.7% for grades 3–4. Frequency of haematological-irAEs seem to be higher with PD-1/PD-L1 inhibitor (4.1 %/ 4.7%) than with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) (0.5%).6 Immune thrombocytopenia represents the most frequent haematological anomaly, occurring in 25%–29% of patients with haematological-irAEs.6 7 Usually, thrombocytopenia occurs early after the initiation of the treatment (median time of 40 days, range 3–405 days).6–16
In our case, thrombocytopenia was severe and life-threatening; such severe thrombocytopenia (with platelet count lower than 5000/mm3) is rarely described with only several cases reported in literature (table 1). In addition, even if platelet count increased to 15 000/mm3 5 days after the introduction of corticosteroids and immunoglobulins, it took 3 months to reach the value of 100 000/mm3 despite daily high-dose methylprednisone, reflecting a slowly reversible irAE.
Table 1.
Review of thrombocytopenia related to immune checkpoint inhibitor
| Ref | Cancer type | ICI agent | Lowest platelet count (mm/3) |
Time to thrombocytopenia diagnosis after ICI | Radiotherapy (prior ICI administration) | irAE treatment | Issue |
| 8 | Lung adenoc. | Nivolumab | 2000 | 15 days | None |
|
Non-fatal |
| 9 | Lung adenoc. | Nivolumab | 2000 | 6 weeks | Yes 30 Gy irradiation (interval NA) |
|
Fatal |
| 10 | Melanoma | Case 1: Nivolumab+Ipilimumab |
<5000 | 15 days | None |
|
Non-fatal |
Case 2:
|
First occurrence, after nivolumab=28 000 Second occurrence, after ipilimumab=1000 |
43 days 10 days |
None Yes, brain SABR (interval NA) |
|
Non-fatal | ||
| Review of 2360 patients treated with ICI: 11 cases with thrombocytopenia | 18 000 to 104 000 Only one with <5000 |
Range from 12 to 173 days |
Unknown | IV steroids (four cases) IVIG in two cases Rituximab in one case |
NA | ||
| 11 | Renal carcinoma cell | Nivolumab+Ipilimumab | 20 000 | 9 days | None | IV Steroids Plasma exchange Rituximab |
Non-fatal |
| 12 | Lung adenoc. | Nivolumab | 5000 | After the sixth injection | None | IV steroids | Non-fatal |
| 13 | Lung adenoc. | Nivolumab | 16 000 | After the second injection | None | IV steroids | Non-fatal |
| 14 | Melanoma | Pembrolizumab | Case 1=1000 Case 2=9000 |
After the first injection After the ninth injection |
None None |
IV steroids IV steroids |
Non-fatal Non-fatal |
Adenoc, adenocarcinoma; ICI, immune checkpoint inhibitor; irAES, immune-related adverse events; IV, intravenous; IVIG, intravenous immunoglobulines; NA, not available; SABR, stereotactic ablative radiotherapy.
Thrombocytopenia appeared lately in the course of nivolumab, after the ninth administration. However, irAEs are known to appear sometimes very late during ICI treatment and even the arrest of immunotherapy; in our case, it is interesting to note that thrombocytopenia appeared rapidly after the administration of nivolumab that closely followed the end of SABR. It is well known that radiotherapy may potentiate the efficacy of immunotherapy via several mechanisms, by inducing apoptosis of tumour cells and thereby increasing cross-presentation of tumour antigens via APC or by increasing the release of more tumour antigens.17 Multiple trials are ongoing for evaluating the benefit for patients to combine SABR and ICIs and the first results showed that this combination seems safe with no difference in AEs among patients who received SABR-ICI combination compared with those who received either modality alone.18 In our case, the fact that the first eight courses of nivolumab did not induce irAE and that thrombocytopenia occurred after the dose of nivolumab that closely followed SABR cannot exclude a potential interaction that could have facilitated the immune-related thrombocytopenia and delayed the complete recovering of toxicity. It is interesting to see that in the previously reported case reports (table 1), one grade 4 and one grade 5 thrombocytopenia occurred in patients that also received previously radiotherapy in a close time relation with ICI.
This case highlights the importance to consider such life-threatening AEs when treating patients with ICIs, to monitor regularly laboratory in these patients and maybe more carefully patients who received ICI rapidly after stereotactic radiotherapy.
Patient’s perspective.
Curiously, the first administrations of nivolumab were OK and rapidly after the end of radiotherapy and the 9th administration of nivolumab, platelets decreased to reach nearly 0. Situation was controlled with corticosteroids but I wonder why this toxicity appeared at this time point?
Learning points.
Thrombocytopenia is a rare immune-related adverse event and may be severe and life-threatening.
High dose of corticosteroids have to be considered early after the diagnosis of immune checkpoint inhibitors (ICIs)-related thrombocytopenia as well as immunoglobulin in severe cases.
The interaction of stereotactic radiotherapy and ICI is not well defined and patients receiving these two modalities in a close time relation should be carefully monitored.
Footnotes
Contributors: AH, A-EY and ES wrote the manuscript. ES and SL corrected the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol 2015;67:4–17. 10.1016/j.molimm.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, McDermott DF, et al. . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxi S, Yang A, Gennarelli RL, et al. . Immune-Related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutros C, Tarhini A, Routier E, et al. . Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13:473–86. 10.1038/nrclinonc.2016.58 [DOI] [PubMed] [Google Scholar]
- 5.Brahmer JR, Lacchetti C, Schneider BJ, et al. . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delanoy N, Michot J-M, Comont T, et al. . Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol 2019;6:e48–57. 10.1016/S2352-3026(18)30175-3 [DOI] [PubMed] [Google Scholar]
- 7.Michot JM, Lazarovici J, Tieu A, et al. . Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer 2019;122:72–90. 10.1016/j.ejca.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 8.Mori H, Sakai C, Iwai M, et al. . Immune thrombocytopenia induced by nivolumab in a patient with non-small cell lung cancer. Respir Med Case Rep 2019;28:100871. 10.1016/j.rmcr.2019.100871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa T, Ozaki Y, Inoue T, et al. . Nivolumab-related severe thrombocytopenia in a patient with relapsed lung adenocarcinoma: a case report and review of the literature. J Med Case Rep 2019;13:316. 10.1186/s13256-019-2245-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiuan E, Beckermann KE, Ozgun A, et al. . Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:8. 10.1186/s40425-017-0210-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youssef A, Kasso N, Torloni AS, et al. . Thrombotic thrombocytopenic purpura due to checkpoint inhibitors. Case Rep Hematol 2018;2018:1–4. 10.1155/2018/2464619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakas Y, Yuce D, Kılıckap S. Immune thrombocytopenia induced by nivolumab in a metastatic non-small cell lung cancer patient. Oncol Res Treat 2017;40:621–2. 10.1159/000477968 [DOI] [PubMed] [Google Scholar]
- 13.Jotatsu T, Oda K, Yamaguchi Y, et al. . Immune-Mediated thrombocytopenia and hypothyroidism in a lung cancer patient treated with nivolumab. Immunotherapy 2018;10:85–91. 10.2217/imt-2017-0100 [DOI] [PubMed] [Google Scholar]
- 14.Le Roy A, Kempf E, Ackermann F, et al. . Two cases of immune thrombocytopenia associated with pembrolizumab. Eur J Cancer 2016;54:172–4. 10.1016/j.ejca.2015.10.073 [DOI] [PubMed] [Google Scholar]
- 15.Davis EJ, Salem J-E, Young A, et al. . Hematologic complications of immune checkpoint inhibitors. Oncologist 2019;24:584–8. 10.1634/theoncologist.2018-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo R. Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front Pharmacol 2019;10:454. 10.3389/fphar.2019.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weichselbaum RR, Liang H, Deng L, et al. . Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol 2017;14:365–79. 10.1038/nrclinonc.2016.211 [DOI] [PubMed] [Google Scholar]
- 18.Hiniker SM, Reddy SA, Maecker HT, et al. . A Prospective Clinical Trial Combining Radiation Therapy With Systemic Immunotherapy in Metastatic Melanoma. Int J Radiat Oncol Biol Phys 2016;96:578–88. 10.1016/j.ijrobp.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]