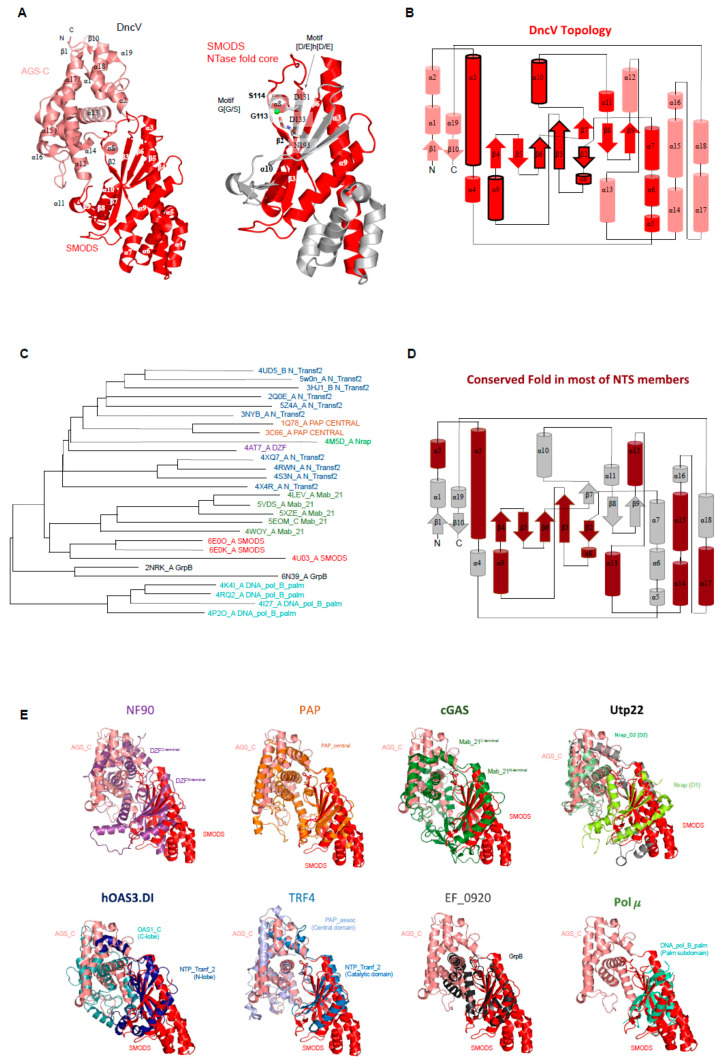
Figure 4.
Structural similarities between the SMODS domain and other nucleotidyltransferase superfamily members. (A) the N-terminal domain SMODS (colored in red) and the C-terminal ACS_C (light pink) of the DncV protein structure are shown as cartoons (PDBID: 4U0M). The SMODS domain is involved in cGAMP synthesis and belongs to the nucleotidyltransferase superfamily (NTS). The NTS fold is characterized by the presence of a minimal conserved core of a mixed β-sheet flanked by α-helices with α1-β1-α2-β2-α3-β3-α4 topology that correspond to α3-β2-α8-β3-α9-β6 (colored in red), missing the α4 element. Various insertions are observed and are colored in grey (right panel). Members of NTS contain three conserved motifs located at the active site: (i) G[G/S] located at α8, (ii) [D/E]h[D/E] (h indicates a hydrophobic amino acid) located at β3, and (iii) [D/E] located at β6. Two of these motifs are conserved in proteins containing SMODS domains (see Figure 5A). (B) topology of DncV, showing the location of the NTS fold core (bold outlines). The secondary structure elements from SMODS are colored in red and the AGS-C domain in light pink. (C) dendrogram showing structures similar to DncV made with the Dali server [79] (query: DncV, PDBID: 4U03). Each domain is colored with different colors and the PDBID_chain and the Pfam name are shown for each branch. (D) The conserved fold found in most of these structures, all NTS members, is shown in brown overlaid on the DncV topology. (E) structural superposition of DncV (PDBID: 4U03) with other NTS members found in the panel (C) dendrogram, using the same colors to represent each domain. At the top of each structural alignment is shown the protein’s name. NF90 is colored in purple (PDBID: 4AT7), PAP is colored in orange (PDBID: 1Q78), cGAS is colored in dark green (PDBID: 5XZE), Utp22 is colored in light green (PDBID: 4M5D), hOAS3.DI is colored in blue (PDBID: 4S3N), TRF4 is colored in light blue (PDBID: 3NYB), EF_0920 is colored black (PDBID: 2NRK), and Pol µ is colored in green (PDBID: 2IHM).