Abstract
Pharmaceutical promotion influences prescribing behavior; these effects, if in the form of market size expansion (i.e. new fills vs. generic or brand substitution) are concerning for second generation antipsychotics (SGA) which are frequently overused. And while most research pertains to individual-level effects, a growing body of work suggests networks of social and professional relationships are important determinants of prescribing behavior. We provide evidence for an association between SGA market size expansion and promotion. We also present new evidence suggesting a meaningful link between promotional activity occurring in professional network and physician network member branded prescribing rates.
Keywords: Medicare, physician behavior, pharmaceutical promotion, social network analysis
Introduction
Pharmaceutical manufacturers spend billions annually on direct-to-physician marketing (e.g. gifts, samples, speaker fees, medical organization funding, travel, meals, lodging, etc.) (CMS, 2017b). Promotional efforts may serve to educate physicians on important developments, new drugs, and side effects. However, conflicts of interest arising from these interactions can influence physician decisions regarding drug choices, leading to higher costs and potentially inappropriate prescribing (Borkowski, Fugh‐Berman, Mullins, & Wood, 2012). Numerous policies have been implemented by professional bodies, states, and the federal government to reduce the risk of negative effects from promotion (Lo, 2009; Mitka, 2010; Office of the Inspector General, 2003). The American Medical Association issued guidelines regarding gifts from industry in 1992, and in 2002 the federal government issued a guidance statement that threatened anti-kick-back prosecution for companies offering gifts intended to promote prescription drug sales.(McMurray, Clarke, Barrasso, & et al., 1991; Office of the Inspector General, 2003) In 2012, the Affordable Care Act required documentation and annual publication of all promotional payments from manufacturers to prescribers, which the Centers for Medicare and Medicaid Services (CMS) publishes in their ‘open payments’ data set.
Evidence for associations between physician-directed promotion and prescribing behavior is found in both the health and health economics literature. For example, Yeh et al. shows higher odds of brand-name statin prescribing in physicians who received payments from industry (Yeh, Franklin, Avorn, Landon, & Kesselheim, 2016) and receipt of promotional payments is shown to be associated with higher per-patient prescribing costs (Perlis & Perlis, 2016). In the health economics literature, findings are consistent, but there is also an attempt to distinguish between substitution vs. market size expansion effects. That is, are physicians simply switching generic to brand, or brand to brand, or are there new prescriptions, that otherwise would not have occurred. Examining the drug famciclovir, Datta and Dave (Datta & Dave, 2017) find evidence only for substitution effects, others have found evidence for both studying different drug classes (David, 2010; Rizzo, 1999; Windmeijer, de Laat, Douven, & Mot, 2006). Prior research, however, has focused on the individual physician in isolation, ignoring any additional impacts exerted through peer interactions. Because physicians do not work in independent silos, their prescribing decisions may not just be a reflection of their own preferences—in fact, there is evidence that the opinions and knowledge prevailing in their professional networks also influences their prescribing decisions (Bae, Nikolaev, Seo, & Castner, 2015; Barnett et al., 2012; Barnett, Landon, O’Malley, Keating, & Christakis, 2011; Cunningham et al., 2012; Fattore, Frosini, Salvatore, & Tozzi, 2009; Gabbay & le May, 2004; Iles, 2001; Keating, Zaslavsky, & Ayanian, 1998; Landon et al., 2013; Landon, Wilson, & Cleary, 1998; Ong, Olson, Chadwick, Liu, & Mandl, 2017; Senge, 1990).
Modern physicians practice alongside other actors (e.g. physician colleagues, nurses, healthcare administrators, payers, pharmaceutical representatives), where any activity may be regarded as a “reaction” to an “action” somewhere else in a complex network of interrelationships (Iles, 2001; Senge, 1990). Landon et al. developed a conceptual model of the effects of health care organizations on health care quality. One of their four proposed domains for organizational attributes that directly influence physician practice behavior was the normative environment (e.g. information sharing, group norms and common practice styles developed through interactions with colleagues) (Landon et al., 1998). The literature on diffusion of innovations highlights similar factors. For example, seminal work by Becker (Becker, 1970) and Coleman (Coleman, 1967) in the 1970s demonstrated the significance of social networks and the influence of local peers on medical technology diffusion. These insights - that provider behavior is strongly influenced by group norms, common practice, and peer interactions – motivate the growing interest in applying social network analysis to answer health services research questions (Bae et al., 2015; Barnett et al., 2012; Barnett et al., 2011; Cunningham et al., 2012; Fattore et al., 2009; Gabbay & le May, 2004; Keating et al., 1998; Landon et al., 2013; Meltzer et al., 2010; Ong et al., 2017). The term social network used is used broadly in the literature an incorporates both social relationship (e.g. friendship networks), as well as professional networks (e.g. physician shared patient networks). Two systematic reviews synthesized evidence demonstrating social networks’ role in shaping clinical decision-making behavior (Bae et al., 2015; Cunningham et al., 2012). Some studies have shown that interactions with and experiences of colleagues, patients, opinion leaders, and pharmaceutical representatives heavily influence clinical decision-making (Gabbay & le May, 2004; Keating et al., 1998); others have found that more dense within-network linkages are useful for improving organization-wide communication.(Meltzer et al., 2010). Several studies have explored issues pertaining to direct to consumer advertising on social network websites (Greene & Kesselheim, 2010; Tyrawski & DeAndrea, 2015), but none that have examined direct to physician marketing.
This literature has typically operationalized networks using surveys and qualitative methods. A validated approach to identify networks using observational data involves the use of shared patient and referral relationships—it has been shown that the likelihood of a true professional relationship between physicians grows with the number of patients shared (Barnett et al., 2011; Landon et al., 2013). In a sufficiently large patient network, pre-existing communities, i.e., naturally occurring local networks not bound by geography or delivery systems factors, can be identified with clustering algorithms (e.g. Louvain community detection algorithm) and used in analysis. Although we are not aware of a scientific literature focused on social networks and drug promotion, the industry posits that pharmaceutical marketing strategies are data-driven; moreover, manufacturers’ own social network analyses likely influences decisions of which physicians to target. For example, Voxx Analytics, which contracts with 19 top manufacturers, highlights the use of social network analysis to identify key relationships, influencers, and opinion leaders,(VOXX Analytics, 2017) which would then allow pharmaceutical companies to embark on data-driven promotional campaigns.
The influence of pharmaceutical promotion on prescribing behavior is of interest for second generation antipsychotics (SGAs). After entering the U.S. market in the 1990s, SGAs saw a dramatic uptake - they are now used in over 90% of all psychiatric related visits (Alexander, Gallagher, Mascola, Moloney, & Stafford, 2011). A known contributor to the steep rise in SGA utilization is off-label use—in fact, estimates suggest that half of all SGA scripts are used for off-label indications (Driessen, Baik, & Zhang, 2016). The frequent and growing use of antipsychotic polypharmacy, i.e., the concurrent use of two or more antipsychotics for extended periods of time, a practice lacking evidence of effectiveness(Barnes & Paton, 2011; Galling et al., 2017; Marchand & Grignon, 2007), has also contributed to the growth in SGA utilization. Because at best, these two practices constitute an inefficient use of resources and at worst their potential for harm exceeds their likely benefit, they are examples of overuse (Orszag, 2008). Hence, it is critical to elucidate the role of pharmaceutical promotion on their growth. (Kreyenbuhl, Valenstein, McCarthy, Ganoczy, & Blow, 2007; Larkin, Ang, Avorn, & Kesselheim, 2014; U.S. Department of Health and Human Services, 2011).
We examine the association between direct-to-physician pharmaceutical promotion and branded second generation antipsychotic prescribing considering both physician-level and network-level effects. We focus on the branded product of the SGA aripiprazole (proprietary name: Abilify), hereafter aripiprazoleBN, one of the costliest drugs overall for Medicaid, and one of the most heavily promoted SGAs. In the U.S. in 2015, Medicaid spending on aripiprazoleBN totaled over $2 billion, second only to the combination drug ledipasvir/sofosbuvir (CMS, 2015), and promotional payments were more than $4 million (CMS, 2017b). We contribute to the literature in two important ways. First, we study representative drug products from an important but understudied drug class: SGAs. At the physician level, we explore both substitution and market expansion effects. This distinction has not traditionally been a focus of the health literature; however, it is key in attempting to determine whether promotional efforts might lead to welfare reducing outcomes such as overuse. For SGAs, overuse would show up primarily in market expansion effects as opposed to substitution. Second, we capitalize on a unique dataset using social network analysis to expand individual-level models to the physician network-level.
Methods
This study is a cross-sectional exploratory analysis linking publicly available Medicare prescriber data, pharmaceutical promotion data, and shared patient networks, in the United States in 2015.
SGA Prescriber Cohort
We define the study cohort as physicians who prescribe one or more of the following SGAs, billed to a Medicare Part D plan, in 2015: Generic: Olanzapine, Quetiapine, Risperidone, Ziprasidone, Brand: Abilify, Seroquel, Invega, Risperdal, Geodon, Zyprexa. These data are available at the provider, drug, year level and includes information on prescriptions such as the quantity, total cost, days supplied, etc. It is derived from CMS’s Chronic Conditions Data Warehouse, which includes records submitted by Medicare Advantage Prescription Drug plans as well as by stand-alone prescription drug plans.(CMS, 2017a) we refer to this as the SGA prescriber cohort.
U.S. healthcare providers are required to obtain a unique 10-digit NPI which identifies them throughout the industry. The CMS National Plan and Provider Enumeration System (NPPES) database tracks active NPIs and includes information such as specialty and practice geography (zip-codes); prescribers include both physician and non-physicians (e.g. nurse practitioners). Because eligible prescribers were drawn from the NPPES, members of the SGA prescriber cohort were required to have a record in the NPPES. We restricted the SGA prescriber cohort to physicians because they are the primary targets of pharmaceutical promotion.
We included non-institutional NPIs in the 50 States. We linked the SGA prescriber cohort to the pharmaceutical promotion data using physician first name, last name and city of practice (the promotional data do not have NPIs). To avoid ambiguous name matching, we removed records where two or more NPIs shared the same first name, last name and city. Analysis of physicians who were excluded for ambiguous name locations revealed some statistically significant differences in specialties, sex and region of provider. We refer to the NPPES NPI population after exclusions as the provider study population. The “physician compare” file assembled by CMS was used to obtain gender and medical school graduation year for physicians. Rurality based on zip-codes was obtained from CMS, and zip-code income and population from the US Census Bureau.
Shared Patient Networks
We used the Care Set Labs 2015 Root NPI graph to generate a complete set of shared patient networks, and then ran clustering algorithms (described below) to identify naturally occurring local networks within the full graph. Similar approaches have been used before, and shared patient networks are a validated means of approximating meaningful physician social networks (Barnett et al., 2011; Landon et al., 2013). As with the SGA cohort, members of shared patient networks were required to have a record in the NPPES. However, unlike the SGA prescriber cohort, we made no restriction on provider type for the creation of networks and thus as a result, nurse practitioners and physician assistants were eligible. While we were interested only in the effects on physicians; we hypothesized that other provider types would still have some network level influence. We linked the shared patient networks to the pharmaceutical promotion with the same method used for the SGA prescriber cohort as described above.
The Root NPI graph is developed using counts of all Medicare beneficiaries shared between two NPIs during 2015; the relationships include both implicit and explicit referrals between physicians, and other provider types.(Trotter, 2017) we assumed undirected edges so that the relationship between provider 1 and 2 is the same as between provider 2 and 1. Once the full network was formed, we used the Louvain community detection algorithm, a method designed to efficiently identify communities in large networks, to group physicians into mutually exclusive local networks (Blondel, 2008). We first partitioned the graph at the state level for two reasons: first, most meaningful professional relationships among physicians are unlikely to occur over state lines due to differences in state and payer policies and insurance plans. Second, doing so allowed for parallel processing to improve computational efficiency when running the clustering algorithm. We also considered a more direct measure of network influence, that is, the promotional activity occurring for a physician’s immediate network neighbors. Communities identified by the Louvain algorithm are mutually exclusive and so do not require that each member is directly connected.
To avoid small cell count estimation issues, we excluded physicians who were the only member of the SGA prescriber cohort in their community (i.e., physicians with no naturally occurring network) and those whose communities were too small (operationalized as n= 2). Inspection of physicians excluded for small community size vs. the final sample, showed statistically significant differences for all characteristics. We use the term ‘community’ to refer to the local clusters identified by the Louvain community detection algorithm.
Pharmaceutical Promotion
We obtained information on payments made by manufacturers to providers from the publicly accessible CMS Open Payments Data Program under which applicable group purchasing organizations and manufacturers are required to submit data about financial relationships, payments, and other transfers of value made to providers.(CMS, 2017b) The unit of observation in the Open Payments data is the transaction, i.e., a payment from a manufacturer to a provider that is associated with one or more promoted drugs. These data are available for the entire calendar year 2015.
Our primary drug of interest was aripiprazoleBN; we sought to identify the influence of promotional payments for aripiprazoleBN on aripiprazoleBN prescribing rates. We also replicated the analyses using branded quetiapineBN product (Seroquel), another heavily promoted and widely prescribed SGA. We matched the promotion data to the prescriber cohort and shared patient networks at the provider (physician name and city), drug, and year levels. Similar approaches to matching has been used previously with these data (DeJong et al., 2016; Singh, Chang, & Rachitskaya, 2017).
Measures
Outcome Measure:
Count of aripiprazoleBN prescriptions filled by each physician in 2015. We analyze the total rate first which picks up both substitution and market size effects. We then condition on the total number of all SGAs (branded and not) filled by each physician in 2015.
Key Independent Variables.
Primary:
We created three measures reflecting the number of promotional payments:
-
(i)
Count of aripiprazoleBN related promotional payments received by each physician, during 2015. Evidence from the literature suggests diminishing returns to promotion, so we also include a quadratic term.
-
(ii)
Count of immediate network neighbors (i.e. directly sharing one or more patients) receiving aripiprazoleBN related payments. We also include a quadratic term here.
-
(iii)
At the community level, we determined the average number of payments received (where a community was determined by the Louvain community detection algorithm). We considered community level effects as both continuous (mean number of payments), categorial (quartiles), and binary (some payments in the community vs. none).
Adjustor variables:
Physician-level variables: Person-level variables were sex, year of medical school graduation, Region (Midwest, Northeast, South, West), rural practice, and specialty. Community-level variables included proportion of male physicians, average graduation year, practice specialty proportions, mean total prescription claim counts.
Network-related variables: We calculated the unweighted (how many physicians they shared patients with) degree of each physician to capture centrality.
Statistical Analysis:
We calculated summary statistics for the SGA prescriber cohort, and community level variables for aripiprazoleBN prescribers using frequencies and means. We estimated a hierarchical Poisson regression model to account for the community level clustering. We assumed that the count of filled aripiprazoleBN scripts for a given physician within a given community arose from a Poisson process with a physician-specific mean, μ. We modeled the log of the expected aripiprazoleBN prescribing rate as a function of the key independent variables and adjustors, as well as a community-specific random effect. We assumed a normally distributed (mean 0 and unknown variance σ2) random effect.
We present four models for each drug, two which capture both market size and substitution effects, and two which can be interpreted as substitution effects. The difference being that we control for total SGA fills in the latter model. We first assess physician level exposure in isolation and then run models which include community level exposure and immediate network neighbor exposure. We calculate elasticities as ((exp(ln(1.01)*β)-1)*100) for easier comparison with the health economics literature and we estimate effects for four promotional payments vs. zero which is slightly above the non-zero mean.
We used SAS proc glimmix to estimate the hierarchical Poisson regression models. We imputed missing values for physician graduation year, median zip-code income and zip-code population using a normally distributed random variable with the sample mean and standard deviation of the respective variables. An ‘NA’ category was included for missing values of sex.
Results
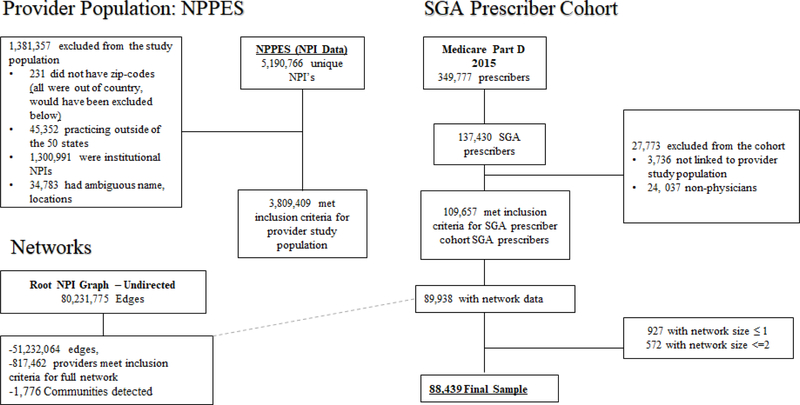
The final study sample included 88,439 physicians nested within 1,776 communities. Figure 1 shows details of the study cohort creation.
Figure 1:
Cohort Creation Flow Chart
SGA Prescriber Cohort Characteristics and Shared Patient Communities:
Of the 88,439 physicians in the final cohort, 24.2% were aripiprazoleBN prescribers, more than half were female, and just under 20% of physicians graduated after 2005 (Table 1). More aripiprazoleBN prescribers were psychiatrists relative to the non-aripiprazoleBN prescriber group.
Table 1:
Physician level characteristics stratified by aripiprazoleBN prescriptions filled in 2015 and community characteristics
| Variable | Value | Did not prescribe aripiprazoleBN (n=67,005) | Prescribed aripiprazoleBN (n=21,434) | p-value |
|---|---|---|---|---|
| Individual Level | ||||
| Sex | Female | 42879 (64.0%) | 13883 (64.8%) | <0.001 |
| Male | 17779 (26.5%) | 5061 (23.6%) | ||
| NA | 6347 (9.5%) | 2490 (11.6%) | ||
| Med School Graduation year >2005 | 11897 (17.8%) | 3686 (17.2%) | 0.062 | |
| Region | Midwest | 16408 (24.4%) | 5904 (27.3%) | <0.001 |
| Northeast | 13962 (20.7%) | 4915 (22.7%) | ||
| South | 23743 (35.2%) | 7596 (35.1%) | ||
| West | 13250 (19.7%) | 3233 (14.9%) | ||
| Rural | 10004 (14.9%) | 3869 (18.1%) | <0.001 | |
| Specialty Description | Internal | 23905 (35.7%) | 4997 (23.3%) | <0.001 |
| Family | 29055 (43.4%) | 6369 (29.7%) | ||
| Neurology | 4720 (7.0%) | 224 (1.0%) | ||
| Psychiatry | 3827 (5.7%) | 8165 (38.1%) | ||
| Other | 5498 (8.2%) | 1679 (7.8%) | ||
| Income, $1,000 (practice zip-code) | median (IQR) | 51.55 (40.51, 69.59) | 49.93 (39.23, 67.77) | <0.001 |
| Total SGA Prescribing Costs | mean (SD) | 2613.22 (7450.63) | 92991.46 (1.6e+05) | <0.001 |
| Total SGA scripts | mean (SD) | 543.67 (464.92) | 880.80 (795.93) | <0.001 |
| Received Payment for aripiprazoleBN | 1046 (1.6%) | 2955 (13.8%) | <0.001 | |
| Received Payment for quetiapineBN | 1012 (1.5%) | 2184 (10.2%) | <0.001 | |
| Number of Payment (>0) for aripiprazoleBN | mean (SD) | 2.68 (6.35) | 5.32 (8.73) | |
| Number of Payment (>0) for quetiapineBN | mean (SD) | 2.31 (7.30) | 4.10 (6.93) | |
| Unweighted Degree, mean (SD) | mean (SD) | 84.96 (99.58) | 89.52 (115.24) | <0.001 |
| Neighbor was paid for aripiprazoleBN | 11821 (17.6%) | 7623 (35.6%) | <0.001 | |
| Mean number of payments | 0.32 (0.96) | 0.86 (1.85) | ||
| Neighbor was paid for quetiapineBN | 10257 (15.3%) | 6302 (29.4%) | <0.001 | |
| Community Level | ||||
| Number of networks | 1,776 | |||
| Community size: full, median (IQR) | 296 (90–640) | |||
| Community size: SGA prescriber cohort, median (IQR) | 33 (12–72) | |||
| Received any aripiprazoleBN payments ** | 1100 (61.94%) | |||
| Received any quetiapineBN payments | 933 (52.53%) | |||
| Mean $ amount paid aripiprazoleBN, mean (SD) | 4.72 (33.02) | |||
| Mean $ amount paid quetiapineBN, mean (SD) | 1.80 (21.99) | |||
| Mean $ amount paid other drugs, mean (SD) | 2,830 (5,653) | |||
| Mean # payments aripiprazoleBN, mean (SD) | 0.05 (0.22) | |||
| Mean # payments quetiapineBN, mean (SD) | 0.03 (0.13) | |||
| Mean # payments Other, mean (SD) | 19.66 (13.77) | |||
| Median graduation year of physicians, mean (SD) | 1995 (1994–1997) | |||
| Proportion of male physicians, mean (SD) | 0.59 (0.11) | |||
| Proportion internal medicine, mean (SD) | 0.09 (0.07) | |||
| Proportion psychiatry, mean (SD) | 0.03 (0.10) | |||
| Proportion cardiology, mean (SD) | 0.02 (0.02) | |||
| Proportion family medicine, mean (SD) | 0.11 (0.10) | |||
| Mean prescription cost for aripiprazoleBN, mean (SD) | 91.56 (210.86) | |||
| Mean prescription cost for any SGA, mean (SD) | 233.26 (610.02) | |||
| Mean prescription cost for quetiapineBN, mean (SD) | 26.76 (67.15) | |||
| Mean prescription cost for other drugs, mean (SD) | 3,922.48 (2,191.15) | |||
The communities contained a median of 296 providers, and a median of 33 physicians belonging to the SGA prescriber cohort. Roughly 62% of the communities received some aripiprazoleBN payments; with a mean payment dollar amount of $16.20.
The mean number of aripiprazoleBN payments was 0.21 (SD: 2), quetiapineBN 0.13 (SD 1.5), and the mean number of network neighbors receiving payments was 0.45 (1.26).
Physician level effects
AripiprazoleBN promotion had a positive and significant association with aripiprazoleBN prescribing rates, with a diminishing effect (negative quadratic term). A one percent change in the number of aripiprazoleBN promotional payments during the year was associated with 0.061% higher prescribing rates [Table 2: Model 0]. The prescribing rate of physicians with four promotional payments was 27% higher than those with 0 [Table 2: Model 0]. Once we constrain the model by total number of SGA promotions (i.e. isolating the substitution effects), the magnitudes smaller; we see physicians with four promotional payments prescribing aripiprazoleBN at a rate that is 12% higher than those with no promotional payments [Table 2: Model 3]. The effect of promotion on prescribing was stronger for quetiapineBN; rates were 51% higher for the 4 vs. 0 promotion groups [Table 3: Model 0]. We saw a similar shift between the market size and substitution effects [Table 3: Model 0, Model 3]. Adding network level promotion variables to our models did not change individual level estimates.
Table 2:
Poisson Regression: Aripiprazol
| N= 88,439 | ||||
|---|---|---|---|---|
| Variable | Market Size + Substitution Effects | Substitution Effects | ||
| Model 0 | Model 1 | Model 2 | Model 3 | |
| Individual level | ||||
| AripiprazoleBN payment count during year, β1 (95% CI) | .0621 (.0614, .0629) | .0628 (.0621, .0636) | .0300 (.0293, .0308) | .0334 (.0326, .0341) |
| AripiprazoleBN payment count during year squared, β2 (95% CI) | −.001 (−.001, −.001) | −.001 (−.001, −.001) | −.000 (−.000, −.000) | −.000 (−.000, −.000) |
| Total SGA Scripts (per 100), β3 (95% CI) | --- | --- | .0927 (.0924, .0929) | .0918 (.0915, .0920) |
| Payment count 4 vs. 0 exp(4β1+ 16β2+) (95% CI) | 1.27 (1.27, 1.27) | 1.27 (1.27, 1.28) | 1.12 (1.12, 1.13) | 1.14 (1.13, 1.14) |
| Elasticity (at 1 vs. 0) | 0.061 | 0.062 | 0.03 | 0.033 |
| Network level | ||||
| Network neighbor received aripiprazoleBN payment, β4 (95% CI) | --- | .1298 (.1276, .1320) | --- | .0420 (.0399, .0441) |
| Network neighbor received aripiprazoleBN payment squared, β5 (95% CI) | --- | −.009 (−.009, −.009) | --- | −.005 (−.005, −.005) |
| Neighbor Payment count 4 vs. 0 exp(4β4+ 16 β5+) (95% CI) | --- | 1.45 (1.44, 1.46) | --- | 1.10 (1.09, 1.10) |
| Any Network aripiprazoleBN payments, β6 (95% CI) | --- | .2747 (.1710, .3784) | --- | .4017 (.2972, .5061) |
| Sigma (SE) | .831 (.034) | .745 (.031) | .727 (.031) | .759 (.032) |
β* represents log rate ratios, exp(β* ) represents rate ratios for specified level,
All models controlled for sex, years since graduation, region, specialty, degree, rurality, zip-code: population, median income, community level: sex, specialty, claim counts, graduation year, random intercept. Model 1 and Model 3 controlled for network size
Table 3:
Poisson Regression: Quetiapine
| N= 88,439 | ||||
|---|---|---|---|---|
| Variable | Market Size + Substitution Effects | Substitution Effects | ||
| Model 0 | Model 1 | Model 2 | Model 3 | |
| Individual level | ||||
| quetiapineBN payment count during year, β1 (95% CI) | .1066 (.1057, .1075) | .0984 (.0973, .0995) | .0800 (.0789, .0812) | .0786 (.0775, .0798) |
| quetiapineBN payment count during year squared, β2 (95% CI) | −.001 (−.001, −.001) | −.001 (−.001, −.001) | −.001 (−.001, −.001) | −.001 (−.001, −.001) |
| Total SGA Scripts (per 100), β3 (95% CI) | --- | --- | .0879 (.0876, .0883) | .0870 (.0867, .0874) |
| Payment count 4 vs. 0 exp(4β1+ 16β2+) (95% CI) | 1.51 (1.50, 1.51) | 1.47 (1.46, 1.47) | 1.36 (1.36, 1.37) | 1.35 (1.35, 1.36) |
| Elasticity (at 1 vs. 0)** | 0.105 | 0.097 | 0.079 | 0.078 |
| Network level | ||||
| Network neighbor received quetiapineBN payment, β4 (95% CI) | --- | .1454 (.1428, .1480) | --- | .0600 (.0564, .0636) |
| Network neighbor received quetiapineBN payment squared, β5 (95% CI) | --- | −.011 (−.011, −.011) | --- | −.006 (−.006, −.006) |
| Neighbor Payment count 4 vs. 0 exp(4β4+ 16 β5+) (95% CI) | --- | 1.49 (1.48, 1.51) | --- | 1.16 (1.14, 1.17) |
| Any Network quetiapineBN payments, β6 (95% CI) | --- | .4397 (.3177, .5617) | --- | .5284 (.4088, .6479) |
| Sigma (SE) | 1.23 (.052) | 1.10 (.047) | 1.17 (.050) | 1.05 (.045) |
β* represents log rate ratios, exp(β* ) represents rate ratios for specified level,
All models controlled for sex, years since graduation, region, specialty, degree, rurality, zip-code: population, median income, community level: sex, specialty, claim counts, graduation year, random intercept. Model 1 andModel 3 controlled for network size
Network level effects
The effect of immediate network neighbor aripiprazoleBN promotion followed a similar pattern to the direct to physician effects: prescribing rates were 45% higher for physicians with four immediate network neighbors receiving aripiprazoleBN payments vs. physicians with no network neighbors receiving aripiprazoleBN payments, and the effect was diminishing (negative quadratic term). Community with some aripiprazoleBN promotional activity vs. communities with none were associated with higher aripiprazoleBN prescribing rates (~50% higher) [Table 2: Model 1]. For the community level effects, the continuous specification was not significant, and there was no discernable dose response pattern for categorical formulations.
Discussion
Linking three national data sets, we quantified the association between pharmaceutical payments to physicians and physician SGA prescribing, focusing on aripiprazoleBN and quetiapineBN, two costly SGA products prone to overuse. We find evidence for both substitution and market size expansion. This is an important finding for researchers and policymakers, as this separation begs the question of whether the new prescriptions resulting from market size expansion are appropriate or not. We also provide the first evidence showing higher prescribing rates when members of a physician’s professional networks are receiving payments, independent of the physicians own relationship with industry.
Our physician level findings are in line with the prior literature. In the range of 0.03 to 0.1, our elasticity estimates come in slightly lower than the summary effect of 0.18 derived in a meta-analysis of 373 econometric estimates of pharmaceutical detailing elasticities (Sridhar, 2014). Our estimates are closer to those found by Datta and Dave (Datta & Dave, 2017) who used physician level data to study famciclovir detailing, with elasticities from fully specified models closer to 0.05. An important difference is they concluded no evidence for market size expansion – only substitution effects. The most comparable study in the health literature, which used the same Open Payments data, examined substitution effects only (DeJong et al., 2016). They found stronger magnitudes of effect for olmesartan (≥4 payments vs. 0: Odds Ratio (OR): 2.26), desvenlafaxine (≥4 payments vs. 0: OR: 2.47), and nebivolol (≥4 payments vs. 0: OR: 2.42); their estimates for Rosuvastatin were more consistent with ours (≥4 payments vs. 0: OR: 1.34).
The responsiveness of prescribing to promotional efforts, and whether behavioral modifications are socially beneficial, neutral, or harmful, depend on drug specific considerations. Unlike direct to consumer advertising which generates new demand by prompting patients to seek new treatments (Chintagunta, 2004), with direct to physician promotion, market size increases must come from more prescriptions with patient population held constant. So, the question becomes, are promotional relationships leading to greater disease awareness and new scripts are written accordingly. Or, are physicians using the drugs for new indications, e.g. off-label, or in combination e.g. polypharmacy. Off-label prescribing is not always clinically problematic, but it is for SGAs, and promotion for off-label indications is always illegal. In the 2000’s there were three major settlements between SGA manufactures and the federal government for illegal off-label SGA promotion (Department of Justice, 2009; Pollack et al., 2014; Wilson, 2010). Under these considerations, our findings of a robust market size expansion effect of SGA promotion is concerning given the frequent overuse of SGAs, mainly in the form of off-label and polypharmacy prescribing (Barnes & Paton, 2011; Carton et al., 2015; Driessen et al., 2016). These practices present a significant burden to our healthcare system. The estimated direct drug costs of off-label use in 2008 was US$6.0 billion; (Alexander et al., 2011) polypharmacy prescribing also represents a financial drain on the system (Valuck et al., 2007). These dollars could be put to better use by budget-constrained public payers. Moreover, these practices put patients at unnecessary risks of serious side-effects including serious cardiometabolic morbidity (Meyer et al., 2008). Some researchers and policy-makers have hinted at the potential influence of pharmaceutical promotion on overuse but the hypothesis is yet to be tested empirically (Kreyenbuhl et al., 2007; Larkin et al., 2014; U.S. Department of Health and Human Services, 2011). While our current analysis does not explicitly answer questions regarding overuse, it provides a strong motivation for further research, as the question of whether physician directed promotion leads to welfare reducing behavior is still unanswered.
Our analysis uncovered several new insights as to how physician networks might amplify associations between pharmaceutical promotion and prescribing. The effects were most apparent when promotional activity of immediate neighbors was considered. Importantly, the addition of the network effects had virtually no impact on our estimates of physician’s own promotional activity, as evidenced by the similar physician-level coefficients in models 0 and 1 and models 2 and 3. This suggests an additional mechanism at play beyond what occurs when physicians take payments directly. The health economics literature generally concludes that information transfer is the dominant mechanism behind direct to physician advertising effects. The effect was less clear at the community level where mean levels (as continuous) of promotion were not significant, and within networks that had some promotional activity, there was no significant relationship with higher levels of promotional effort. Therefore, while the higher prescribing rates found in networks with some promotional activity is a strong and statistically significant effect, we are more cautious in our interpretation given the lack of a dose response relationship. Because we are not aware of prior research on the prescribing effects of pharmaceutical promotion mediated by prescribers’ professional networks, we cannot directly compare these findings to extant evidence. However, our results are consistent with evidence on the effects of physicians’ normative environment on their prescribing behavior. For example, using administrative data, Fattore et al (Fattore et al., 2009) found that general practitioners working in a collaborative arrangement had similar prescribing behavior. Ong et al found that improved care cohesion within networks lowered dangerous prescribing (Ong et al., 2017). Moreover, our findings are consistent with literatures that have evaluated the effects of social networks on a broad array of behaviors, for example, obesity spreading through social ties (Christakis & Fowler, 2007), and network structure being significantly associated with a hospital’s patterns of patient care.(Barnett et al., 2012).
Our analyses have several limitations. First, our data are cross-sectional, and aggregated to the physician -drug - year – level, and thus, our findings should be interpreted as associative and not causal. Additionally, we cannot parse out temporal confounding. For example, the most probable explanation for the stronger effects seen with quetiapineBN vs. aripiprazoleBN is that the FDA approved aripiprazole for generic marketing at the end of April 2015. During 2015, over 28,000 generic aripiprazole scripts were filled and there was a reduction in aripiprazoleBN scripts of around 17,000 during 2015 compared to 2014 (CMS part D data). Promotion for aripiprazoleBN trailed off during the year as well (5,398 scripts in January 2015 vs. 1,878 in Dec 2015). Because certain pharmacists have the authority to substitute branded for generic drugs, the number of scripts we see filled in 2015 for aripiprazoleBN are likely less than the number of scripts written. The effect of promotion, therefore, may be underestimated in this study for aripiprazoleBN. In addition, unmeasured confounding variables may have biased our results and since name and location were used to match payments data, there is still the possibility of matching inaccuracies. The clustering algorithm was applied once using standard parameters; parameter tuning might be a productive exercise for future research. Next, the study reflects the effects of promotion on SGA prescribing for Medicare beneficiaries by Medicare-billing physicians and thus, the generalizability to Medicaid or commercial insurance is uncertain.
Conclusion
The literature used to inform conflict of interest policy related to pharmaceutical promotion still has an important unanswered question: whether resultant prescribing increases are socially harmful. While the answer will vary by drug, our research points towards welfare reducing effects for SGAs. Furthermore, current results may underestimate the influence of promotion on physician prescribing decisions by not considering the residual effects of promotion in physicians’ communities; highlighting the importance of physicians’ normative environments and imply that efforts to improve prescribing quality might not need to reach all members of a network.
Budget-constrained payers such as Medicaid and Medicare looking to lower drug costs should consider further action to limit pharmaceutical promotion. They should also consider the role or physicians’ normative environments and the diffusion of influence through professional networks as they attempt to curb overuse. Future research on physician prescribing decisions might also consider the network-level effects demonstrated in this study.
Acknowledgements:
I gratefully acknowledge Dr. Marcela-Horvitz-Lennon, and Dr. Sharon-Lise Normand for their support and mentorship, reviews, and methodological advice. Additionally, I would like to acknowledge Lisa Jonsson, Michele Abbot, Sara Turner, Sujeong Park, Rouslan Karimov, PhuongGiang Nguyen, and Dr. Dmitry Khodyakov for their helpful comments.
Financial support for this study was provided in part by grants from the National Institute of Mental Health (R01-MH106682), the National Institute of Minority Health and Health Disparities (R01-MDO12428), and the Anne and James Rothenberg Dissertation Award. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Research involving Human Participants and/or Animals / Informed Consent: This study uses publicly available, secondary data and was approved by the RAND Human Subjects Protection Committee.
This study was approved by the RAND Human Subjects Protection Committee.
Footnotes
Conflicts of interest: None
REFERENCES
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, & Stafford RS (2011). Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf, 20(2), 177–184. doi: 10.1002/pds.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Nikolaev A, Seo JY, & Castner J. (2015). Health care provider social network analysis: A systematic review. Nurs Outlook, 63(5), 566–584. doi: 10.1016/j.outlook.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Barne TR., & Pato C. (2011). Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs, 25(5), 383–399. doi: 10.2165/11587810-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Barnett ML, Christakis NA, O’Malley J, Onnela JP, Keating NL, & Landon BE (2012). Physician patient-sharing networks and the cost and intensity of care in US hospitals. Med Care, 50(2), 152–160. doi: 10.1097/MLR.0b013e31822dcef7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett ML., Landon BE., O’Malley AJ., Keating NL., & Christakis NA. (2011). Mapping physician networks with self-reported and administrative data. Health Serv Res, 46(5), 1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MH (1970). Factors affecting diffusion of innovations among health professionals. Am J Public Health Nations Health, 60(2), 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel VD Jean-Loup G; Lambiotte Renaud; Lefebvre Etienne. (2008). Fast unfolding of communities in large networks. J. Stat. Mech (2008) P10008. [Google Scholar]
- Borkowski L, Fugh‐Berman A, Mullins P, & Wood SF (2012). Impacts of Pharmaceutical Marketing on Healthcare Services in the District of Columbia: Focus on Antipsychotics in Children. Retrieved from [Google Scholar]
- Carton L, Cottencin O, Lapeyre-Mestre M, Geoffroy PA, Favre J, Simon N, … Rolland B. (2015). Off-Label Prescribing of Antipsychotics in Adults, Children and Elderly Individuals: A Systematic Review of Recent Prescription Trends. Curr Pharm Des, 21(23), 3280–3297. [DOI] [PubMed] [Google Scholar]
- Chintagunta M. a. (2004). Responsiveness of Physician Prescription Behavior to Salesforce Effort: An Individual Level Analysis. Marketing Letters, 15(2/3), 129–145. [Google Scholar]
- Christakis NA, & Fowler JH (2007). The spread of obesity in a large social network over 32 years. N Engl J Med, 357(4), 370–379. doi: 10.1056/NEJMsa066082 [DOI] [PubMed] [Google Scholar]
- CMS. (2015). Medicaid Drug Spending Dashboard. Retrieved from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Dashboard/2015-Medicaid-Drug-Spending/2015-Medicaid-Drug-Spending.html
- CMS. (2017a). Medicare Provider Utilization and Payment Data: Part D Prescriber. Retrieved from https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/part-d-prescriber.html
- CMS. (2017b). Open Payments Public Use Files: Methodology Overview & Data Dictionary. Retrieved from https://www.cms.gov/OpenPayments/Downloads/OpenPaymentsDataDictionary.pdf
- Coleman JS, Katz E. and Menzel H. (1967). Medical Innovation: A Diffusion Study. Inidianapolis, The Bobbs-Merril Company, Inc. [Google Scholar]
- Cunningham FC, Ranmuthugala G, Plumb J, Georgiou A, Westbrook JI, & Braithwaite J. (2012). Health professional networks as a vector for improving healthcare quality and safety: a systematic review. BMJ Qual Saf, 21(3), 239–249. doi: 10.1136/bmjqs-2011-000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, & Dave D. (2017). Effects of Physician-directed Pharmaceutical Promotion on Prescription Behaviors: Longitudinal Evidence. Health Econ, 26(4), 450–468. doi: 10.1002/hec.3323 [DOI] [PubMed] [Google Scholar]
- David GM, Richards-Shubik S, S. (2010). The Effects of Pharmaceutical Marketing and Promotion on Adverse Drug Events and Regulation. American Economic Journal: Economic Policy, 2, 1–25. [Google Scholar]
- DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, & Dudley RA (2016). Pharmaceutical Industry-Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries. JAMA Intern Med, 176(8), 1114–1110. doi: 10.1001/jamainternmed.2016.2765 [DOI] [PubMed] [Google Scholar]
- Department of Justice. (2009). Eli Lilly and Company Agrees to Pay $1.415 Billion to Resolve Allegations of Off-label Promotion of Zyprexa. Retrieved from https://www.justice.gov/archive/opa/pr/2009/January/09-civ-038.html
- Driessen J, Baik SH, & Zhang Y. (2016). Trends in Off-Label Use of Second-Generation Antipsychotics in the Medicare Population From 2006 to 2012. Psychiatr Serv, 67(8), 898–903. doi: 10.1176/appi.ps.201500316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore G, Frosini F, Salvatore D, & Tozzi V. (2009). Social network analysis in primary care: the impact of interactions on prescribing behaviour. Health Policy, 92(2–3), 141–148. doi: 10.1016/j.healthpol.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Gabbay J., & le May A. (2004). Evidence based guidelines or collectively constructed “mindlines?” Ethnographic study of knowledge management in primary care. BMJ, 329(7473), 1013. doi: 10.1136/bmj.329.7473.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galling B, Roldan A, Hagi K, Rietschel L, Walyzada F, Zheng W, … Correll CU (2017). Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry, 16(1), 77–89. doi: 10.1002/wps.20387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JA, & Kesselheim AS (2010). Pharmaceutical marketing and the new social media. N Engl J Med, 363(22), 2087–2089. doi: 10.1056/NEJMp1004986 [DOI] [PubMed] [Google Scholar]
- Iles VS, Kim (2001). Organisational Change A Review for Health Care Managers, Professionals and Researchers National Coordinating Centre for NHS Service Delivery and Organisation, London. [Google Scholar]
- Keating NL, Zaslavsky AM, & Ayanian JZ (1998). Physicians’ experiences and beliefs regarding informal consultation. JAMA, 280(10), 900–904. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl JA, Valenstein M, McCarthy JF, Ganoczy D, & Blow FC (2007). Long-term antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patterns. Psychiatr Serv, 58(4), 489–495. doi: 10.1176/ps.2007.58.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Onnela JP, Keating NL, Barnett ML, Paul S, O’Malley AJ, … Christakis NA (2013). Using administrative data to identify naturally occurring networks of physicians. Med Care, 51(8), 715–721. doi: 10.1097/MLR.0b013e3182977991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, & Cleary PD (1998). A conceptual model of the effects of health care organizations on the quality of medical care. JAMA, 279(17), 1377–1382. [DOI] [PubMed] [Google Scholar]
- Larkin I, Ang D, Avorn J, & Kesselheim AS (2014). Restrictions on pharmaceutical detailing reduced off-label prescribing of antidepressants and antipsychotics in children. Health Aff (Millwood), 33(6), 1014–1023. doi: 10.1377/hlthaff.2013.0939 [DOI] [PubMed] [Google Scholar]
- Lo BF,MJ. (2009). Conflict of interest in medical research, education, and practice. Institute of Medicine; Washington, DC. [PubMed] [Google Scholar]
- Marchand J, & Grignon S. (2007). A case-control study of patients with resistant schizophrenia after clozapine discontinuation. J Clin Psychopharmacol, 27(1), 90–92. [DOI] [PubMed] [Google Scholar]
- McMurray RJ, Clarke OW, Barrasso JA, & et al. (1991). Gifts to physicians from industry. JAMA, 265(4), 501–501. doi: 10.1001/jama.1991.03460040077034 [DOI] [PubMed] [Google Scholar]
- Meltzer D, Chung J, Khalili P, Marlow E, Arora V, Schumock G, & Burt R. (2010). Exploring the use of social network methods in designing healthcare quality improvement teams. Soc Sci Med, 71(6), 1119–1130. doi: 10.1016/j.socscimed.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, … Lieberman JA (2008). Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res, 101(1–3), 273–286. doi: 10.1016/j.schres.2007.12.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M. (2010). Program seeks to enlist physicians to flag questionable promotion of drugs. JAMA, 304(1), 28–29. doi: 10.1001/jama.2010.861 [DOI] [PubMed] [Google Scholar]
- Office of the Inspector General. (2003). Compliance Program Guidance for Pharmaceutical Manufacturers. Retrieved from https://oig.hhs.gov/fraud/docs/complianceguidance/042803pharmacymfgnonfr.pdf
- Ong MS, Olson KL, Chadwick L, Liu C, & Mandl KD (2017). The Impact of Provider Networks on the Co-Prescriptions of Interacting Drugs: A Claims-Based Analysis. Drug Saf, 40(3), 263–272. doi: 10.1007/s40264-016-0490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Overuse, Underuse, and Misuse of Health Care, (2008).
- Perlis RH, & Perlis CS (2016). Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs. PLoS One, 11(5), e0155474. doi: 10.1371/journal.pone.0155474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack EC., Wang H., Bekelman JE., Weissman G., Epstein AJ., Liao K., … Armstrong K. (2014). Physician social networks and variation in rates of complications after radical prostatectomy. Value Health, 17(5), 611–618. doi: 10.1016/j.jval.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo J. (1999). Advertising and Competition in the Ethical Pharmaceutical Industry: The Case of Antihypertensive Drugs. The Journal of Law & Economics, 42(1), 89–116. [Google Scholar]
- Senge P. (1990). The Fifth Discipline: the art and practice of the learning organisation. London: Doubleday/Century Business. [Google Scholar]
- Singh N, Chang JS, & Rachitskaya AV (2017). Open Payments Database: Anti-Vascular Endothelial Growth Factor Agent Payments to Ophthalmologists. Am J Ophthalmol, 173, 91–97. doi: 10.1016/j.ajo.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Sridhar SM, Murali K; Albers Sönke (2014). Pharmaceutical Detailing Elasticities: A Meta-Analysis In Ding Min E.J. a.S.S. (Ed.), Innovation and Marketing in the Pharmaceutical Industry, Emerging Pactices, Research, and Policies. New York: Springer. [Google Scholar]
- Trotter F (2017). CareSet Labs Releases Root NPI Graph – a Greatly Improved Medicare Doctor Referral Dataset. Retrieved from https://careset.com/rootnpigraph/
- Tyrawski J, & DeAndrea DC (2015). Pharmaceutical companies and their drugs on social media: a content analysis of drug information on popular social media sites. J Med Internet Res, 17(6), e130. doi: 10.2196/jmir.4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2011). Medicare Atypical Antipsychotic Drug Claims for Elderly Nursing Home Residents (OIG publication No. OEI-07-08-00150). Retrieved from Washington, DC: : http://oig.hhs.gov/oei/reports/oei-07-08-00150.pdf.
- Valuck RJ, Morrato EH, Dodd S, Oderda G, Haxby DG, Allen R, & Medicaid Pharmacotherapy Research, C. (2007). How expensive is antipsychotic polypharmacy? Experience from five US state Medicaid programs. Curr Med Res Opin, 23(10), 2567–2576. doi: 10.1185/030079907X233214 [DOI] [PubMed] [Google Scholar]
- VOXX Analytics. (2017). VOXX Products. Retrieved from http://www.voxxanalytics.com/products
- Wilson D. (2010). Side Effects May Include Lawsuits. New York Times; Retrieved from http://www.nytimes.com/2010/10/03/business/03psych.html?pagewanted=all [Google Scholar]
- Windmeijer F, de Laat E, Douven R, & Mot E. (2006). Pharmaceutical promotion and GP prescription behaviour. Health Econ, 15(1), 5–18. doi: 10.1002/hec.1007 [DOI] [PubMed] [Google Scholar]
- Yeh JS, Franklin JM, Avorn J, Landon J, & Kesselheim AS (2016). Association of Industry Payments to Physicians With the Prescribing of Brand-name Statins in Massachusetts. JAMA Intern Med, 176(6), 763–768. doi: 10.1001/jamainternmed.2016.1709 [DOI] [PubMed] [Google Scholar]