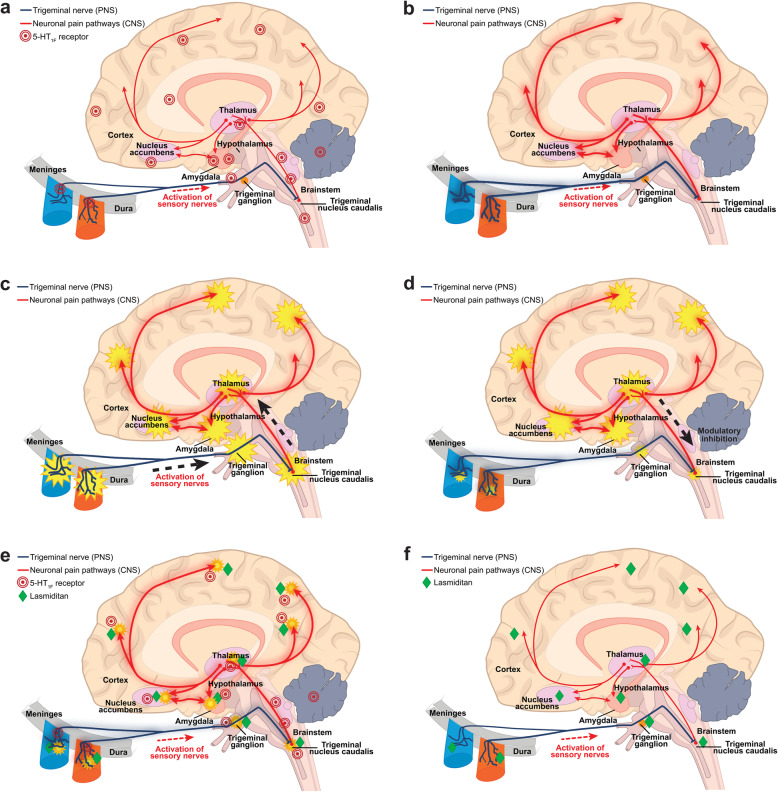
Fig. 1.
a Trigeminal and pain pathways associated with pain signaling during a migraine attack plus 5-HT1F receptor locations in PNS and CNS areas involved in the pathophysiology of migraine. b Activation of trigeminal and pain pathways during a migraine attack; sustained or repeated activation of pain pathways leads to increasing sensitivity of trigeminal neurons in the brain stem. c Activated nerves release various substances including neuropeptides and neurotransmitters such as CGRP and glutamate that can exacerbate neurogenic inflammation and nociceptor pain signaling in migraine; this may hyperexcite neurons to propagate pain responses and enhance central sensitization. d In addition to other endogenous inhibitory processes, serotonin from pain modulating pathways activates 5-HT receptors that can inhibit neuropeptide and neurotransmitter release, thereby inhibiting their local activity and downstream neuronal signaling; however, these modulatory mechanisms may be disturbed in migraine pathophysiology, predisposing patients with migraine to an attack. e Lasmiditan crosses the blood-brain barrier and presumably acts in both the PNS and the CNS to selectively activate 5-HT1F receptors to inhibit the release of neuropeptides and neurotransmitters such as CGRP and glutamate, thereby inhibiting their local activity and migraine attack pain pathways. f Preclinical and ex vivo human evidence suggests that lasmiditan inhibits PNS trigeminal nerve and CNS pain signaling pathways, exerting therapeutic effects in the treatment of migraine without causing vasoconstriction. Abbreviations: CNS, central nervous system; mRNA, messenger ribonucleic acid; PNS, peripheral nervous system. Note: 5-HT1F receptor locations are based on human and animal mRNA, immunohistochemistry, and functional biology studies