Table 2. Hydrogenation Reactor Model Equation and Boundary Conditions14,24,25,29.
| mass balance equation | formula |
|---|---|
| gaseous compounds (i = H2, H2S) | 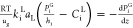 |
| gaseous compounds in liquid phase (i = H2, H2S) | 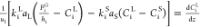 |
| organic sulfur in liquid phase | 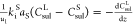 |
| consumption or production by chemical reaction for H2, H2S, and organic sulfur | kH2SaS(CH2 – CH2S) = ρBηHDSrHDSkH2SaS(CH2SL – CH2S) = – ρBηHDSrHDSksulSaS(Csul – CsulS) = ρBηHDSrHDS |
| boundary conditions | CH2L(z = 0) = CH2(initial) CH2SL(z = 0) = 0 Csul(z = 0) = CsulL(initial) PH2(z = 0) = PH2G(initial) PH2S(z = 0) = 0 |
| Chemical reaction rates equations | |
| HDS | 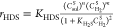 |
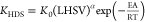 | |
