Fig 5.
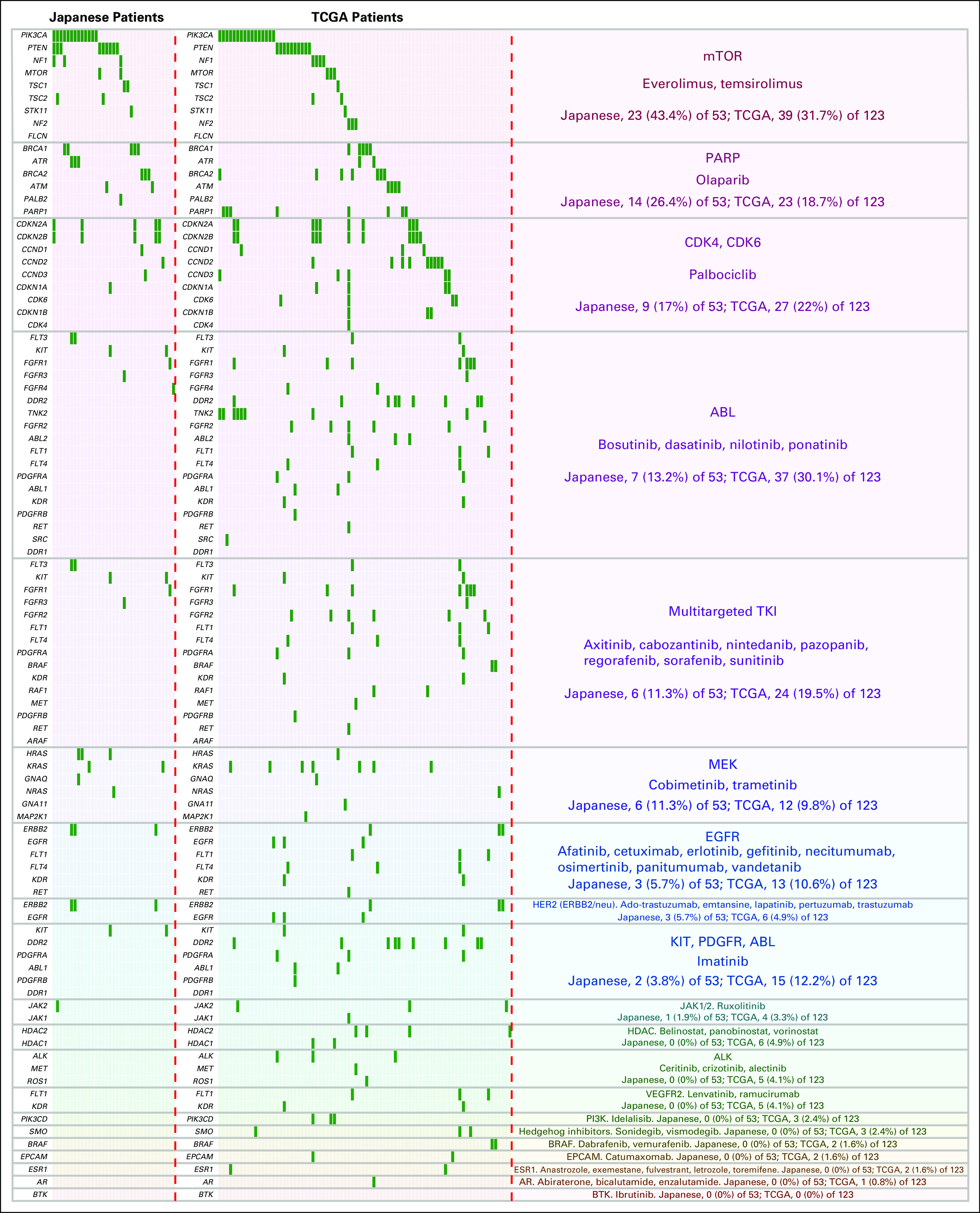
Genetic alterations in Japanese patients with triple-negative breast cancer and patients in The Cancer Genome Atlas (TCGA) cohort potentially targetable by US Food and Drug Administration (FDA) –approved cancer therapies. The genes associated with an FDA-approved drug (on or off label) are listed, and each individual genetic alteration is indicated as a green bar. The FDA-approved cancer therapies are shown in the right column. The alteration rates in the Japanese population (n = 53; alterations, 35 [66%] of 53) and TCGA cohort (n = 123; alterations, 82 [66.7%] of 123) are also shown in the right column. AR, androgen receptor; BTK, Bruton tyrosine kinase; EGFR, epidermal growth factor receptor; EPCAM, epithelial cell adhesion molecule; HDAC, histone deacetylase; mTOR, mammalian target of rapamycin; PARP, poly (ADP-ribose) polymerase; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol 3-kinase; TKI, tyrosine kinase inhibitor; VEGFR2, vascular endothelial growth factor receptor 2.
