To the Editor: The polymyxin antibiotics colistin (polymyxin E) and polymyxin B (PMB) became available in the 1950s; thus, they did not undergo contemporary drug development procedures. Their clinical use has recently resurged, assuming an important role as salvage therapy for otherwise untreatable gram-negative infection.[1] Multidrug-resistant (MDR) bacterial infections are primarily caused by gram-negative bacilli (GNB), such as Pseudomonas aeruginosa and Klebsiella pneumoniae, in lung transplant (LT) patients.[2] The increasing prevalence of MDR-GNB infections in LT patients has led to an upsurge in the use of these “older” drugs. During the treatment course, we should pay attention to severe and life-threatening respiratory paralysis caused by neuromuscular blockage associated with PMB. Herein, we report a rare case of a 67-year-old LT patient who suffered from respiratory arrest induced by PMB.
A 67-year-old male with a healthy past history underwent left single LT for end-stage idiopathic pulmonary fibrosis in October 2018. All anesthetics and sedatives were discontinued 3 days after the operation. Anti-microbial prophylaxis was administered according to the standard practice in our hospital, including cefoperazone-sulbactam/piperacillin-tazobactam, vancomycin, ganciclovir, sulfadiazine and trimethoprim tablets, caspofungin and inhaled amphotericin, while vancomycin was stopped 4 days later. Immunosuppressive regimens included oral tacrolimus, mycophenolate mofetil, and methylprednisolone. Low-molecular-weight heparin was used for prophylactic anti-coagulant therapy. He was transferred from the intensive care unit to our ward on the third day post-operation. He could walk around without the assistance of oxygen. On the 14th day, the patient had an episode of productive cough with leukocytosis and elevated procalcitonin. Chest X-ray showed moderate infiltrate in the left lower lobe. Endoscopic findings revealed endobronchial erythema and purulent excretions with positive culture of carbapenem-resistant Acinetobacter baumannii (CRAB), which was sensitive only to PMB (minimum inhibitory concentration [MIC] ≤0.5 μg/mL) and tigecycline (MIC = 2 μg/mL). The patient's serum creatinine level was 112.9 μmol/L (35.0–106.0 μmol/L) before the anti-microbial regimen combining PMB (25,000 IU/kg, divided into two doses) and tigecycline (100 mg, q12h) [Figure 1].
Figure 1.
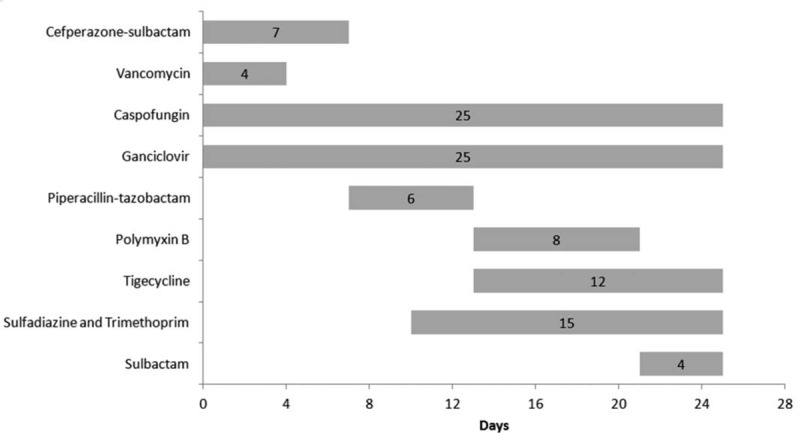
All the anti-microbial agents used in the post-operative period in a 67-year-old male patient who underwent left single lung transplant.
Two days after the initiation of intravenous PMB, he had slight chest tightness and weakness in both lower limbs. On the third day, he experienced sudden hypercapnic respiratory failure (pH 7.004, PaCO2 83.6 mmHg, and PaO2105 mmHg on fraction of inspiration O2 [FiO2] 0.5) 0.5 h after the start of the infusion of PMB. He was intubated quickly after the failure of non-invasive positive pressure ventilation. He recovered soon and was extubated within 24 h. However, he developed the same episode during the infusion 8 days after the initiation of intravenous PMB. Bronchoscopy, cranial computed tomography, X-ray, echocardiogram, electrocardiogram, and laboratory tests excluded pulmonary embolism, pneumothorax, airway congestion, electrolyte disturbance, and nervous system disease such as stroke and epilepsy. Each hypercapnic respiratory failure occurred suddenly, accompanied by dyspnea, weakness in the lower limbs and diminished breath sounds on auscultation. An ultrasound of the diaphragm muscle indicated that the bilateral diaphragm muscle was thinned with decreased muscle motion amplitude. The patient later recalled that at the time of the crisis, he was fully aware but felt unable to breathe. We discussed with the pharmacist all the drugs that the patient took before respiratory distress. PMB was absolutely determined to be the cause of respiratory distress given that it is a drug that can induce neuromuscular blockade. Thereafter, PMB was discontinued, and the patient was extubated again without any sequelae. Sulbactam was substituted for PMB and combined with tigecycline for the treatment of CRAB infection. He was discharged 1.5 months after LT operation without any recurrence of respiratory distress.
In the recent decade, there has been an increasing prevalence of MDR-GNB infections, especially in critical patients, and antibiotics are becoming less effective against MDR bacteria. Infections caused by these so-called superbugs are associated with high mortality because therapeutic options are limited. PMB has been used as the last-resort therapy for the treatment of severe GNB infections in a variety of locations.[3,4]
Respiratory muscle paralysis is a rare but potentially fatal complication of the use of polymyxin. Our patient experienced sudden respiratory arrest twice, which required intubation and mechanical ventilation after PMB infusions, as well as lower limb muscle weakness, which was consistent with the neurotoxicity of PMB. The Naranjo Adverse Drug Reaction Probability Scale score in our patient was 9, which indicated a definite relationship between the administration of PMB and respiratory arrest. In addition, PMB-associated respiratory failure was suspected to be associated with diaphragmatic dyspraxia due to neuromuscular blockade. It is worth noting that there is no specific time window of complications. Some complications occur after a single dose of infusion, whereas others occur after receiving antibiotics for 45 days.[5] Nearly 70% of patients recover eventually, suggesting that neuromuscular blockade caused by PMB is reversible. The drug dosage before respiratory failure is also variable.[3]
We analyzed the cause of PMB-induced neuromuscular blockade: (1) PMB was newly approved by the Chinese Food and Drug Administration in September 2017 and was introduced by China-Japan Friendship Hospital (China) in March 2018. Thus, a lack of clarity remains about how to utilize and dose of PMB optimally. (2) At that time, we did not set up therapeutic drug monitoring. Thereafter, according to international consensus guidelines, we accepted an area under the curve 24-h target of 50 to 100 mg·h/L as a toxicity standpoint.[1] (3) Our patient had slight renal dysfunction, which may have increased the toxicity of PMB. Previous studies suggested that the most important risk factor for polymyxin-associated neuromuscular blockade was renal insufficiency.[5] (4) The patient received a variety of drugs after LT operation. However, he discontinued all the anesthetics at least 10 days before the initiation of intravenous PMB with continuing immunosuppressors, corticosteroids, anti-microbial drugs and protective drugs, and we do not know the exact interaction between the above drugs and PMB. (5) PMB was infused over 1 h in the patient, while prolonging the infusion time over 2 h in LT patients with a variety of concomitant drugs required further investigation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form of the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Funding
This work was supported by the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (No. 2019PT320020).
Conflicts of interest
None.
Footnotes
How to cite this article: Chen WH, Lin L, Wang XX, Kong XD, Guo LJ, Zhao L, Liang CY, Xing B, Cao B, Wang C, Chen JY. Respiratory arrest associated with polymyxin B in a lung transplant patient. Chin Med J 2020;133:1375–1377. doi: 10.1097/CM9.0000000000000826
Wen-Hui Chen and Lan Lin contributed equally to this work.
References
- 1.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019; 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiddon AR, Dawson KL, Fuentes A, Perez KK, Peterson LE, Kaleekal T. Postoperative antimicrobials after lung transplantation and the development of multidrug-resistant bacterial and Clostridium difficile infections: an analysis of 500 non-cystic fibrosis lung transplant patients. Clin Transplant 2016; 30:767–773. doi: 10.1111/ctr.12746. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha A, Soriano SM, Song MSC. Intravenous colistin-induced acute respiratory failure: a case report and a review of literature. Int J Crit Illn Inj Sci 2014; 4:266–270. doi: 10.4103/2229-5151.141487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares DS, Reis ADF, Silva Junior GBD, Leite TT, Parente Filho SLA, Rocha CVO, et al. Polymyxin-B and vancomycin-associated acute kidney injury in critically ill patients. Pathog Glob Health 2017; 111:137–142. doi: 10.1080/20477724.2017.1309338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wunsch H, Moitra VK, Patel M, Dzierba AL. Polymyxin use associated with respiratory arrest. Chest 2012; 141:515–517. doi: 10.1378/chest.11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]


