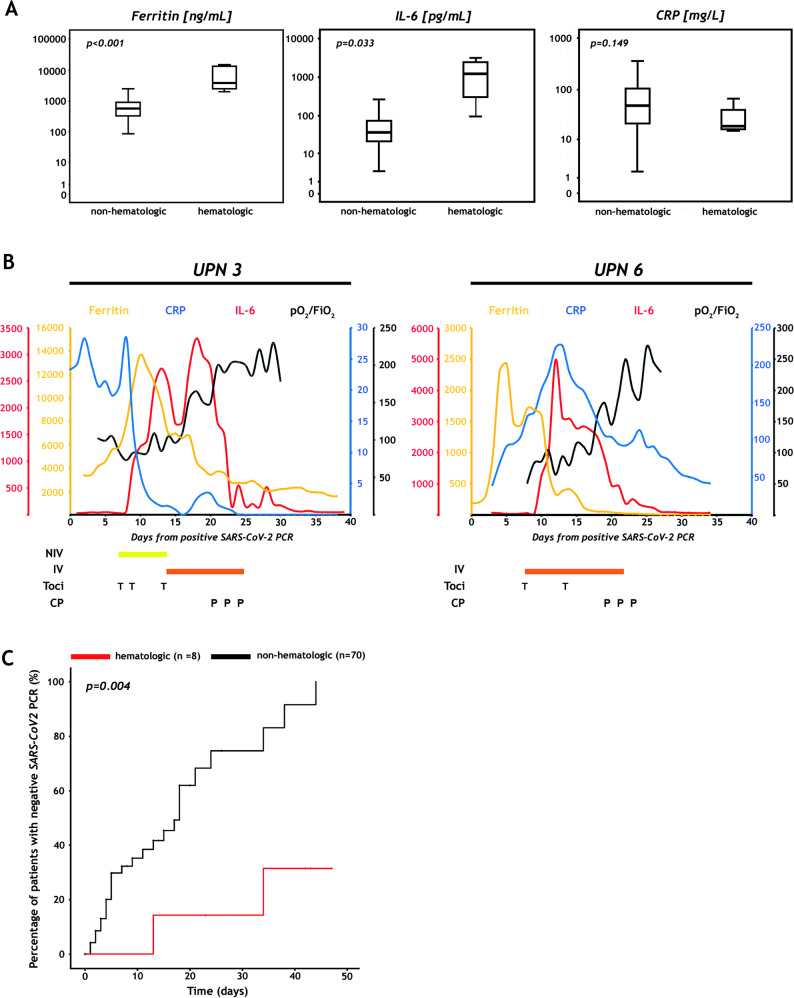
Fig. 1. Inflammatory markers, clinical courses after SARS-CoV-2 convalescent plasma administration and time to negative nucleic acid test in patients with hematologic malignancies and COVID-19.
a Comparison of inflammatory markers in patients with and without hematologic malignancies. Box plots display serum ferritin (left), interleukin-6 (IL-6; middle), and C-reactive protein (CRP; right) in eight hematologic versus 70 non-hematologic patients on log-transformed y-axis. The maximum values were used in every subject, except in patients who received tocilizumab. Here, the maximum values before tocilizumab infusion were selected because serum ferritin and IL-6 would regularly increase, and CRP would decrease after tocilizumab administration. P-values were calculated with the Kruskal–Wallis test (see Supplementary information). b Clinical courses of patients UPN3 and UPN6 receiving SARS-CoV-2 convalescent plasma. Serum ferritin (ng/mL), CRP (mg/L), IL-6 (pg/mL) and the ratio of partial pressure of oxygen in blood (PaO2 in millimeters of mercury) and the fraction of oxygen in the inhaled air (FiO2) are depicted as a measure of inflammation and respiratory function, respectively. Tocilizumab was administered at a dose of 8 mg/kg body weight. Patients received 200 mL of ABO compatible SARS-CoV-2 convalescent plasma (CP) every other day for three times. CP was collected by standard apheresis and further pathogen-inactivated by INTERCEPT Blood System (Cerus, B.V. Europe). IV invasive mechanical ventilation, NIV non-invasive ventilation, P convalescent plasma, T tocilizumab, UPN unique patient number. c Time to SARS-CoV-2 qRT-PCR negativity in hematologic and non-hematologic patients. Analysis was performed with competing risk cumulative incidence estimators and Gray’s tests (see Supplementary information). Data cut off was May 12, 2020. The black line indicates non-hematologic patients, the red line patients with hematologic diseases.