Abstract
Background
Experimental studies provide evidence of the harmful effect of human papillomavirus (HPV) infection on pregnancy, but observational studies are inconclusive. We systematically assessed the association between HPV and adverse pregnancy outcomes.
Methods
We searched electronic databases up to December 1, 2019. We included observational studies on the association between HPV and adverse pregnancy outcomes. We conducted a random-effect meta-analysis for each outcome and assessed heterogeneity between studies.
Results
From 3034 citations, we included 38 studies and quantitatively synthesized 36 studies. Human papillomavirus was significantly associated with preterm birth (age-adjusted odds ratio [aOR], 1.50; 95% confidence interval [CI], 1.19–1.88), preterm premature rupture of membranes (aOR, 1.96; 95% CI, 1.11–3.45), premature rupture of membranes (aOR, 1.42; 95% CI, 1.08–1.86), intrauterine growth restriction (aOR, 1.17; 95% CI, 1.01–1.37), low birth weight (aOR, 1.91; 95% CI, 1.33–2.76), and fetal death (aOR, 2.23; 95% CI, 1.14–4.37). No significant association was found for spontaneous abortion (aOR, 1.14; 95% CI, 0.40–3.22) and pregnancy-induced hypertensive disorders (aOR, 1.24; 95% CI, 0.80–1.92). Most of the studies were of moderate or low quality, and substantial between-studies heterogeneity remained unexplained.
Conclusions
We found a consistent and significant association between HPV and preterm birth and preterm premature rupture of membranes. Human papillomavirus may also be associated with intrauterine growth restriction, low birth weight, and fetal death, but findings are limited by suboptimal control of biases.
Keywords: adverse pregnancy outcomes, human papillomavirus, meta-analysis, pregnant women, systematic review
This meta-analysis suggests that HPV is associated with preterm birth and preterm premature rupture of membranes. Associations are also possible for other adverse pregnancy outcomes, but the potential biases and the small number of studies do not allow definitive conclusions.
Infections and changes in vaginal microbiota during pregnancy are garnering substantial attention as potential causes of adverse pregnancy outcomes [1]. In vitro and animal experiments suggest that human papillomavirus (HPV) can complete full replication cycle in trophoblasts and thereby cause inhibition of blastocyst formation [2], failure of endometrial implantation [3], and apoptosis of embryonic cells [4]. The placental abnormalities observed in vitro could translate in vivo into several forms of adverse pregnancy outcomes, such as spontaneous abortion [5], preterm birth (PTB) [4], or pregnancy-induced hypertensive disorders (PIHDs) [6]. However, findings from observational studies are equivocal.
Three previous reviews summarized the literature on the association between HPV and adverse pregnancy outcomes [7–9]. However, those reviews did not adequately address potential bias and thus provided a limited understanding of the association between HPV and adverse pregnancy outcomes. Therefore, we conducted a systematic review and meta-analyses to (1) estimate the strength of association between HPV exposure and adverse pregnancy outcomes and (2) assess the extent of confounding and inconsistency within the current literature.
METHODS
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [10] and has been registered in International Prospective Register of Systematic Reviews (PROSPERO; number CRD42016033425).
Information Source and Search Strategy
We searched MEDLINE (PubMed and Ovid interfaces), EMBASE, and EBM Reviews from inception to December 1, 2019. The Supplementary File 1 provides the full search strategy. We also hand-searched reference lists of included titles and previous reviews.
Eligibility Criteria
We included all types of observational studies without language restriction, provided that there was an English or French abstract. The exposure of interest was HPV infection, measured directly (HPV-deoxyribonucleic acid [DNA]) or indirectly (HPV-related lesions) in all genital sites (vulva, vagina, or cervix) and placenta. We excluded studies that explored the association between cervical surgical treatment and adverse pregnancy outcomes. The primary outcomes were spontaneous abortion and PTB. Secondary outcomes included PIHD, premature rupture of membranes (PROMs), preterm PROMs (PPROM), low birth weight (LBW), intrauterine growth restriction (IUGR), and fetal death. The details on definition and prioritization of adverse pregnancy outcomes were described in the review protocol [11].
Study Selection and Data Extraction
Two reviewers (J.N. and N.Z.) independently and in duplicate screened titles/abstracts and extracted data from selected full-text reports, using a predesigned form [11]. Disagreements were resolved by discussion with coauthors (H.T. and M.-H.M.). Study characteristics were summarized within Supplementary Table 1.
Quality Assessment and Risk of Bias Across Studies
We described the confounding variables according to the adjustment method used in each study. We assessed the overall study quality based on the potential of selection bias, exposure misclassification, and confounding using a modified Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies [12]. Contour-enhanced funnel plots were used to explore the potential of publication bias. We conducted cumulative meta-analyses to examine the effect of study size on the pooled estimates.
Data Synthesis
For each primary outcome, we computed a pooled crude odds ratio (OR) and 95% confidence interval (CI) from raw data using DerSimonian and Laird (D + L) random-effects models. We used forest plots to represent the dispersion of observed OR. The I2 statistic with its 95% CI were used to quantify the proportion of variance in observed ORs that reflected the true heterogeneity between studies. Weighted pooled age adjusted ORs (aORs) were also estimated for each outcome using only studies that adjusted at least for age. We further conducted subgroup analyses when possible comparing pooled ORs from studies that adjusted and did not adjust for each of these characteristics: multiple pregnancies, genital infections, obstetrical risk factors, history of adverse pregnancy outcome, socioeconomic status, parity/gravidity, tobacco, and/or drug use. We also conducted subgroup analyses according to the following study’s characteristics: different time point for HPV measure, type of specimen used, study quality, study’s population setting, type of HPV testing, and geographic location. We conducted sensitivity analyses to assess robustness of our findings by restricting pooled estimate to high-quality studies, HPV exposure during pregnancy, or HPV-DNA testing. All statistical analyses were conducted using STATA (version 14.3; College Station, TX).
RESULTS
Study Selection
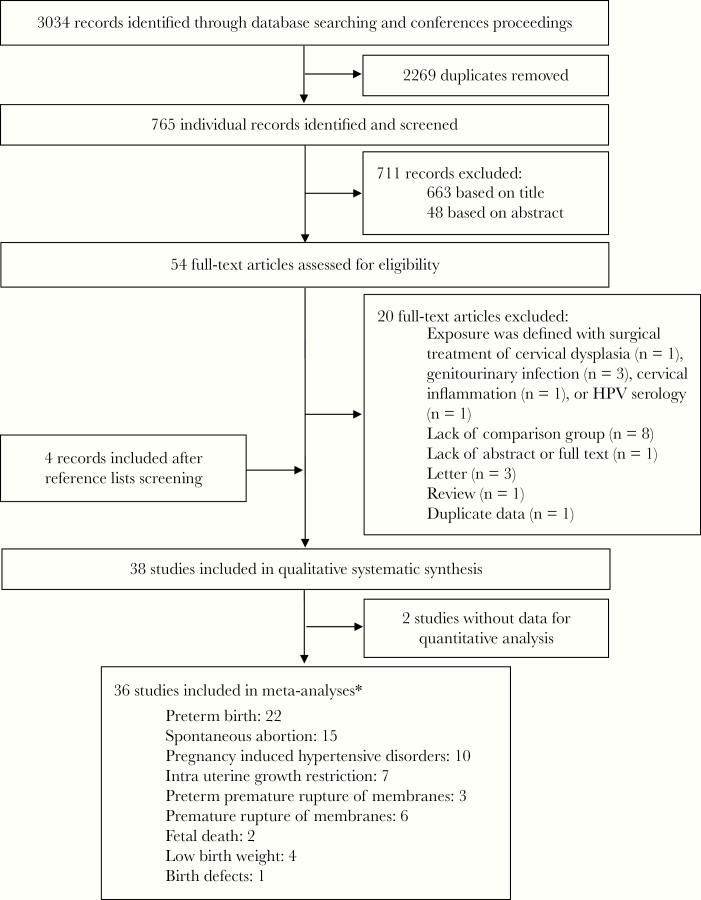
Our search yielded 3034 citations, of which 765 remained after removing duplicates. We retained 38 studies that fulfilled the inclusion criteria [4–6, 13–47]. Two studies without raw data were excluded from the quality assessment and quantitative synthesis [31, 37]. Thirteen studies included more than one adverse pregnancy outcome [4, 6, 13, 17, 21, 26, 30, 38, 39, 41, 42, 46, 47] (Figure 1).
Figure 1.
Study flow diagram. *, Some studies included more than 1 adverse pregnancy outcome.
Study Characteristics
Exposure
Human papillomavirus exposure was measured using HPV testing in 26 studies [4, 5, 13, 15–20, 22, 23, 25, 27, 29, 31–37, 39, 43–46]. Exposure was based on concurrent testing for HPV and cervical cytology (HPV/Pap-cotesting) in 4 studies [6, 14, 26, 42]. In the remaining studies, abnormal cytology alone [20, 24, 28, 38, 40, 41] or presence of genital warts during pregnancy [21, 30, 47] were considered as a proxy for HPV infection. One study in particular had 2 distinct samples based on cytology alone and HPV-DNA testing [20]. Human papillomavirus was identified in cervical or cervicovaginal samples [6, 14–18, 20, 22, 24–26, 28, 31–38, 40–45] or in placenta specimens [4, 5, 13, 19, 23, 27, 29, 39, 45]. Two studies collected both cervical and placental specimens [23, 45].
Primary Outcomes
Twenty-two studies [4, 6, 13, 14, 17, 20–22, 24, 26, 28–31, 38, 39, 41–44, 46, 47] and 1 subsamples [20] reported on PTB. All, except for 5 studies [13, 30, 31, 42, 46], provided definition of PTB as delivery before 37 weeks of gestation. Only 1 study mentioned that gestational age was estimated based on the first day of last menstrual period [29].
Spontaneous abortion was reported in 16 studies [5, 15, 16, 18, 19, 25, 27, 32–37, 39, 45, 46]. Five studies specified the spontaneous abortion as a pregnancy loss before 20 weeks of gestation [5, 15, 16, 19, 45], whereas 9 studies did not provide a duration definition [18, 25, 32–37, 46]. The remaining 2 studies included some cases of spontaneous abortion between 14 and 23 [27] or 8 and 22 gestational weeks [39].
Secondary Outcomes
Seven studies reported on IUGR [13, 23, 26, 30, 38, 40, 46]. Of these, 4 studies provided definition of IUGR as birth weight below 5th percentile [13] or 10th percentile for gestational age [13, 23, 38, 40]. The remaining 3 studies did not provide any definition [26, 30, 46].
The PIHDs were reported in 10 studies [4, 6, 13, 17, 26, 30, 38, 41, 46, 47]. Pre-eclampsia was the only PIHD reported in 7 studies [4, 6, 13, 17, 38, 41, 47], whereas 3 studies included several hypertensive disorders [26, 30, 46].
Four studies [21, 30, 41, 47], including 3 large population-based cohorts [21, 30, 47], explored the association between HPV exposure and LBW. All studies defined LBW as birth weight under 2500 grams.
Six studies reported on rupture of membranes before the onset of labor (PROM) [17, 26, 30, 38, 41, 47], and 3 studies included rupture of membranes that occurred before 37 weeks of gestation and before the onset of labor (PPROM) [38, 42, 46].
Fetal death and birth defects were reported in 2 retrospective cohorts [13, 26] and 1 population-based case-control study [21], respectively. Supplementary Table 1 provides details of included studies.
Quality of Individual Studies
We scored 13 studies [13, 14, 22, 26, 27, 29, 33, 38–40, 42–44], 20 studies [4, 6, 15–25, 28, 30, 32, 34, 35, 41, 46, 47], and 3 studies [5, 36, 45] as being at low, moderate, or high risk, respectively, of bias (Supplementary Figure 1).
The most important limit was the lack of control for potential confounders. Multivariate regressions were used to control for confounding in studies on PTB [14, 21, 26, 28, 38, 41, 42, 44, 47], spontaneous abortion [27, 32, 33], or secondary adverse pregnancy outcomes [6, 17, 21, 26, 38, 40–42, 47]. The remaining studies were grouped according to the presence of other methods of adjustment. First, there was a group of studies that used restriction and excluded women with conditions predisposing to adverse pregnancy outcomes, such as multiple pregnancies, hypertensive disorders, gestational diabetes, or concomitant infections. A second group of studies used bivariate analysis showing similar distributions for specific variables. Finally, one study on birth defects matched infants according to sex, gestational age, and parents’ residence [21]. Three studies used none of above-mentioned methods [5, 36, 45]. Supplementary Table 2 provides the summary of confounders considered as appropriately adjusted and describes the methods used to control for confounding in each study.
QUANTITATIVE SYNTHESIS
Primary Outcomes
Preterm Birth
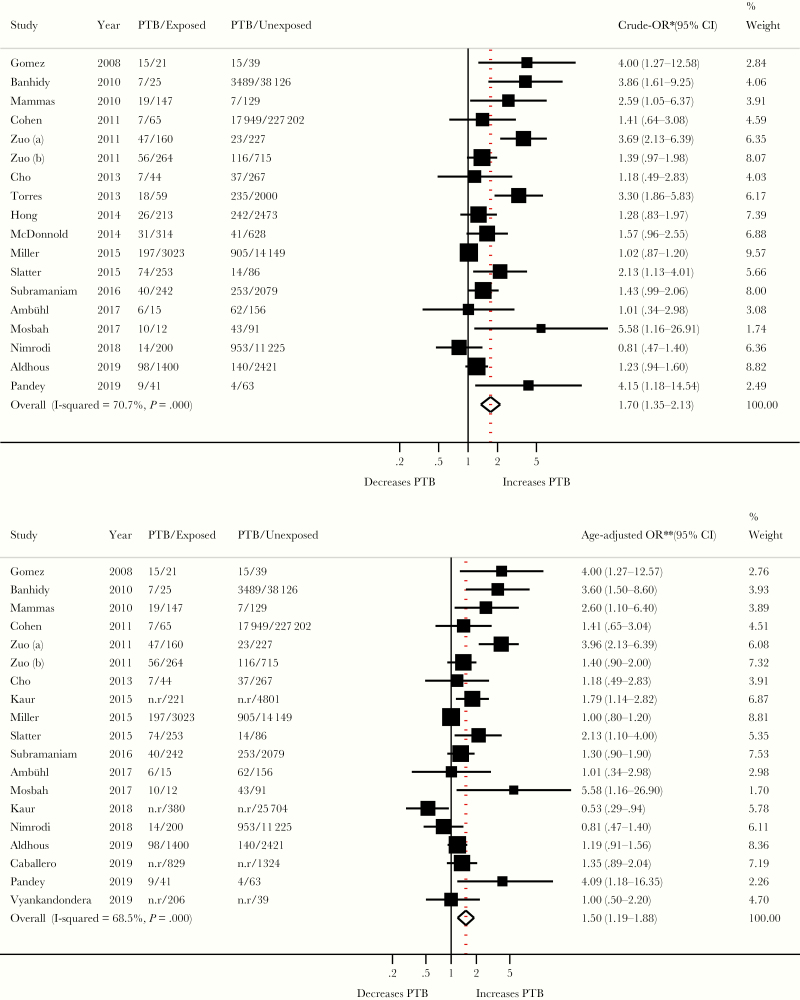
Both overall crude OR (1.70; 95% CI, 1.35–2.13; I2 = 71%; n = 18) and pooled age aOR (1.50; 95% CI, 1.19–1.88; I2 = 68%; n = 19) showed a significant association between HPV exposure and PTB (Figure 2). The observed study-specific ORs ranged from 0.81 to 5.58. A substantial proportion of this variability was due to true heterogeneity between studies rather than chance (I2 = 71%; 95% CI, 53%–82%; P < .001). The subgroup analyses provided estimates with largely overlapping CIs (Figure 3). Table 1 shows the impact of restricting analysis to populations with specific characteristics. The overall age aOR increased as a result of the restriction on studies that measured HPV exposure using HPV testing (aOR, 2.01; 95% CI, 1.33–3.03) or studies that detected HPV during pregnancy (aOR, 1.70, 95% CI, 1.06–2.73). The overall estimate was still positive and significant (aOR, 1.32; 95% CI, 1.06–1.65) when restricting the analysis to the 9 studies at low risk of bias.
Figure 2.
Association between human papillomavirus (HPV) infection and preterm birth (PTB). *, Study’s crude odds ratios (ORs) were derived from study’s raw data and size weighted pooled using random-effects model. Studies without raw data were not included in pooled crude synthesis. **, Study’s ORs adjusted for at least maternal age were size weighted and pooled using random-effects model. Refer to the Supplementary Table 2 for more details on variables adjusted for in each study. CI, confidence interval; n.r, not reported.
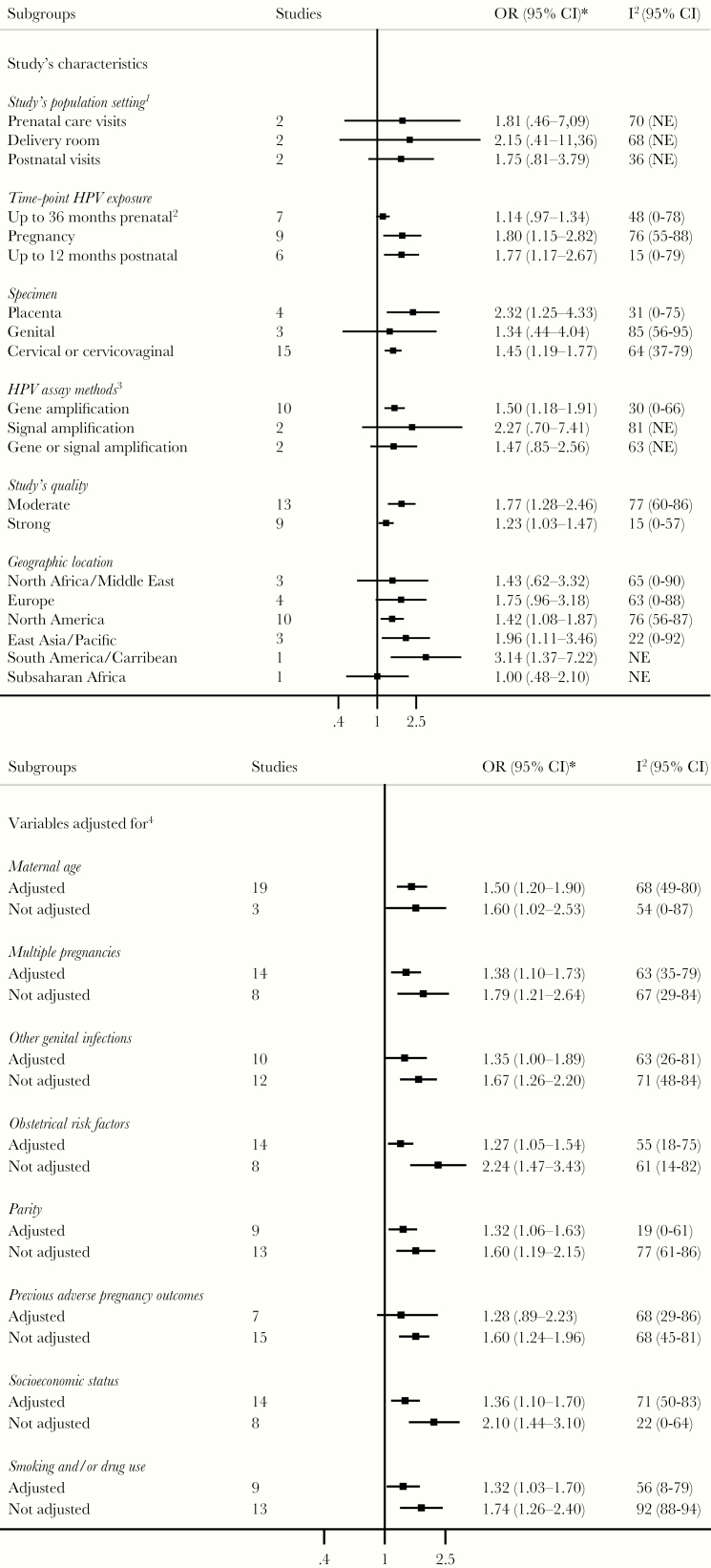
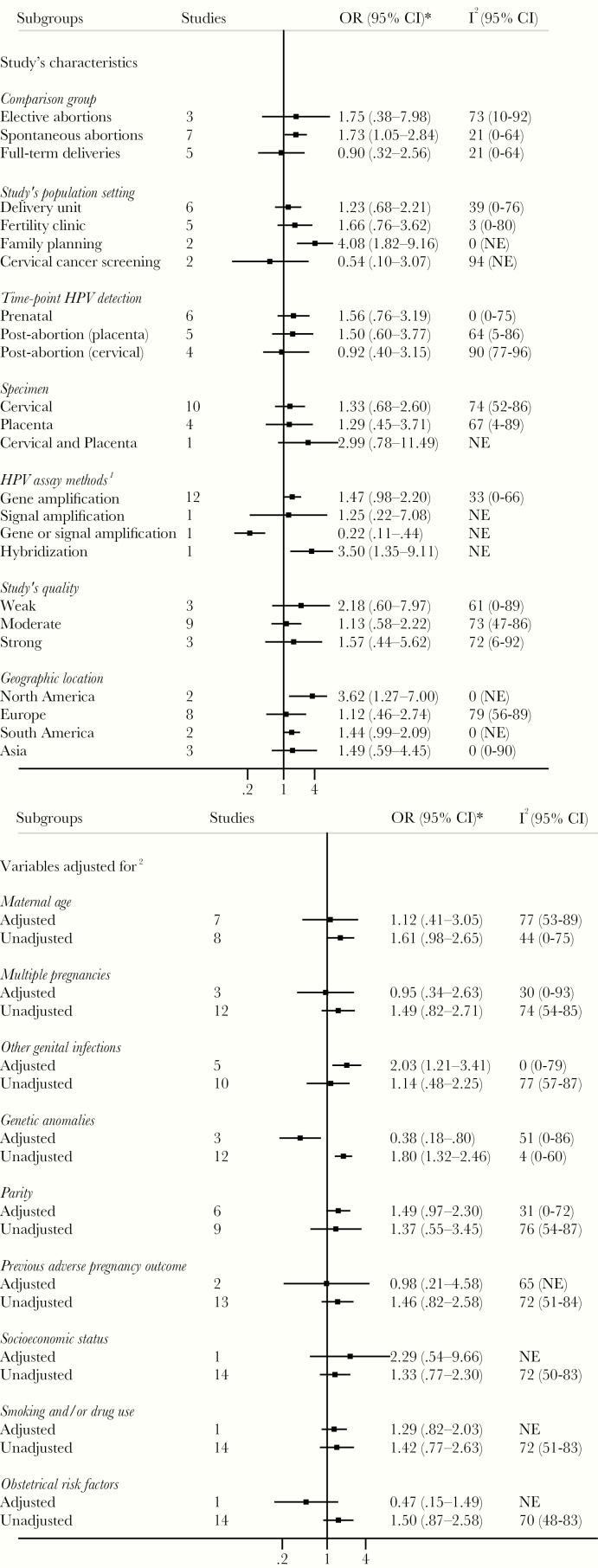
Figure 3.
Subgroup analyses of association between human papillomavirus (HPV) infection and preterm birth. *, Subgroup odds ratios (ORs) are size weighted and pooled individual study ORs, using random-effects model including studies that adjusted for at least the subgroup characteristic. Not adjusted subgroup ORs are size weighted and pooled individual study ORs using random-effects model including studies that did not adjusted for the subgroup characteristic. 1, Only for prospective cohorts and prospective case-controls studies. 2, Human papillomavirus exposure may have been measured during pregnancy in some studies, but details were not provided. 3, Refer to Supplementary Table 1 for more details on laboratory methods used in each study. 4, Refer to Supplementary Table 2 for more details on adjustment methods used and variables adjusted for in each study. I2, percentage of the variability in ORs that is due to true heterogeneity across subgroup’s studies. CI, confidence interval; NE, not estimable.
Table 1.
Sensitivity Analyses of Association Between HPV Infection and Preterm Birth
| Sensitivity Analysis | Rationale | Number of Studies | OR (95% CI)a | I2 (95% CI) |
|---|---|---|---|---|
| HPV exposure measurement: HPV DNA testing | Reducing misclassification of HPV exposure through including only studies that detected HPV by HPV-DNA testing | 10 | 2.01 (1.33–3.03) | 66 (34–83) |
| HPV exposure measurement: HPV DNA or HPV-cotestingb | Reducing misclassification of HPV exposure by including only studies that used HPV-DNA or HPV-cotesting | 12 | 1.77 (1.31–2.38) | 61 (26–79) |
| HPV exposure measurement: HPV DNA testing and low risk of biasc | Reducing misclassification of HPV exposure and confounding by including only studies that used HPV-DNA testing and adjusted for at least 4 of the following confounders: maternal age, multiple pregnancies, other genital infections, obstetrical risk factors, parity, preterm birth history, smoking, and socioeconomic status | 6 | 1.54 (1.04–2.28) | 44 (0–78) |
| HPV exposure during pregnancy | Assuming that pregnancy represents the exposure time- window, the inclusion of only studies that measured HPV exposure during pregnancy should ensure the construct validity underlying the association between HPV and adverse pregnancy outcomes | 8 | 1.70 (1.06–2.73) | 78 (56–89) |
| Low risk of bias | Reducing confounding by including only studies that adjusted for at least 4 of the following confounders: maternal age, multiple pregnancies, other genital infections, obstetrical risk factors, parity, preterm birth history, smoking, and socioeconomic status | 9 | 1.32 (1.06–1.65) | 33 (0–69) |
Abbreviations: CI, confidence interval; DNA, deoxyribonucleic acid; HPV, human papillomavirus; OR, odds ratio.
aThe pooled ORs are the random-effect, weighted summary of individual study ORs included in each sensitive analysis and included only study that adjusted for at least maternal age.
bExposure was based on concurrent testing for HPV and cervical cytology. Refer to Supplementary Table 1 for details on HPV detection methods used by each study.
cRefer to Supplementary Table 2 for the summary of confounders considered as appropriately adjusted for and the methods used to control for confounding in each study.
Spontaneous Abortion
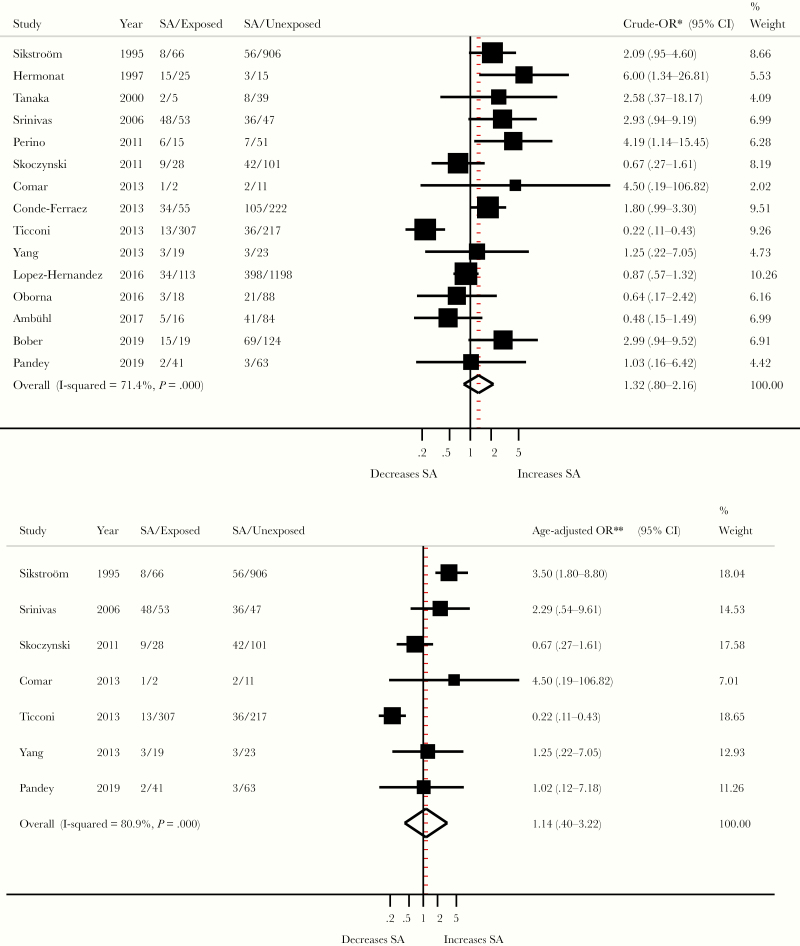
There was no significant association between HPV and spontaneous abortion, as assessed by the overall crude (OR, 1.32; 95% CI, 0.80–2.16; I2 = 71%; n = 15) or age aOR (1.14; 95% CI, 0.40–3.22; I2 = 81%; n = 7) (Figure 4). Most subgroup analyses according to the main potential confounders and study characteristics yielded similar summary estimates with largely overlapping CIs (Figure 5).
Figure 4.
Association between human papillomavirus infection and spontaneous abortion (SA). *, Study’s crude odds ratios (ORs) were derived from study’s raw data and size weighted pooled using random-effects model. **, Study’s ORs adjusted for at least maternal age were size weighted pooled using random-effects model. Refer to Supplementary Table 2 for more details on variables adjusted for in each study. CI, confidence interval.
Figure 5.
Subgroup analyses of association between human papillomavirus (HPV) infection and spontaneous abortion. *, Subgroup odds ratios (ORs) are size weighted and pooled individual study OR, using random-effects model including studies that adjusted for at least the subgroup characteristic. Not adjusted subgroup ORs are size weighted and pooled individual study ORs using random-effects model including studies that did not adjusted for the subgroup characteristic. 1, Refer to Supplementary Table 1 for more details on laboratory methods used in each study. 2, Refer to the Supplementary Table 2 for more details on adjustment methods used and variables adjusted for in each study. CI, confidence interval; I2, percentage of the variability in ORs that is due to true heterogeneity across subgroup’s studies; NE, not estimable.
Secondary Outcomes
Intrauterine Growth Restriction
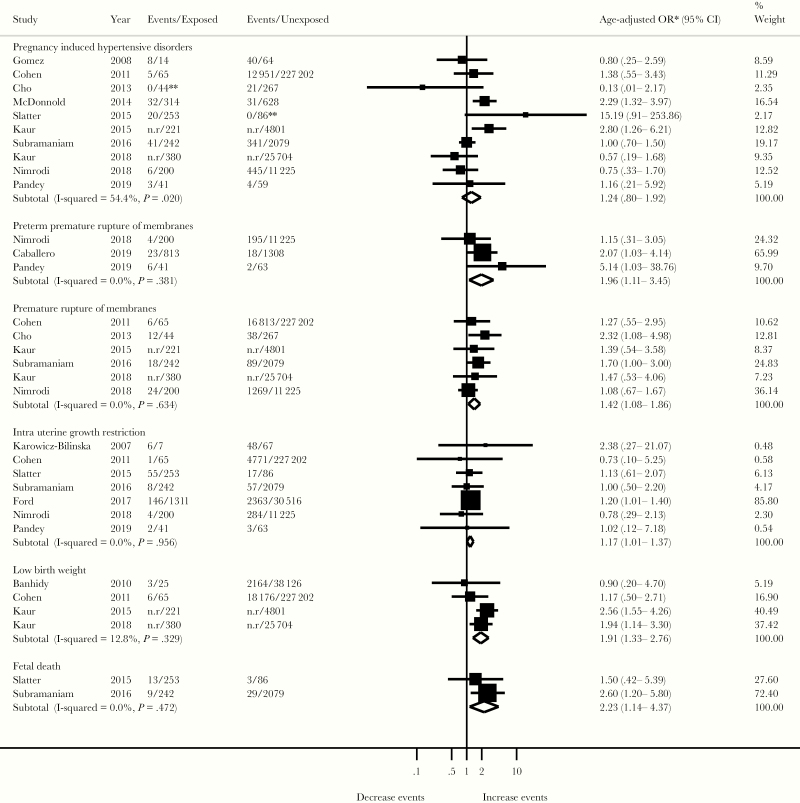
Overall, HPV exposure was associated with IURG (aOR, 1.17; 95% CI, 1.01–1.37; I2 = 0%, n = 7). This overall estimate was largely conditional on 1 population-based study of low risk of bias that contributed 66% of all 222 HPV-exposed IUGR cases [40] (Figure 6).
Figure 6.
Association between human papillomavirus infection and adverse pregnancy outcomes. *, For each adverse pregnancy outcome, the summary effect is a random-effect, weighted odds ratio (OR) derived from individual study OR adjusted for at least maternal age. Refer to the Supplementary Table 2 for details on variables adjusted for in each study. **, If no events were observed in one of the comparison groups, .5 was added to each cell of 2 × 2 table. CI, confidence interval; n.r, not reported.
Pregnancy-Induced Hypertensive Disorders
The pooled association between HPV exposure and PIHD was not significant (aOR, 1.24; 95% CI, 0.80–1.92; I2 = 54%; n = 10) (Figure 6). In addition to age, 5 of 10 studies adjusted for ethnicity, smoking, or chronic high blood pressure [6, 26, 38, 41, 47], and 2 reported a significant association between prenatal HPV exposure and pre-eclampsia [6, 41].
Low Birth Weight
Overall, HPV exposure was significantly associated with LBW (aOR, 1.91; 95% CI, 1.33–2.76; I2 = 13%; n = 4) (Figure 6). However, these studies were of low quality, because HPV exposure was approximated by prenatal abnormal cytology [41] or genital warts [21, 30, 47]. Of these studies, 2 large studies of low quality accounted for 78% of overall weight [41, 47]. Their exclusion changed the strength and significance of the overall estimate (aOR, 1.10; 95% CI, 0.52–2.32; I2 = 0%; n = 2).
Premature Rupture of Membranes/ Preterm Premature Rupture of Membranes
Human papillomavirus exposure was significantly associated with PROM (aOR, 1.42; 95% CI, 1.08–1.86; I2 = 0%; n = 6). The pooled estimate of PPROM (aOR, 1.96; 95% CI, 1.11–3.45; I2 = 0%; n = 3) was largely influenced by one study with low risk of bias, which contributed 70% of all HPV-exposed PPROM and controlled for multiple confounders including concurrent genital infections [42].
Fetal Death
Association between HPV exposure and fetal death was assessed in 2 studies [13, 26]. Of these studies, 1 had a large sample size (n = 2321), and extensively controlled for potential confounders by restriction and multivariate analysis, and thus had a low risk of bias [26]. This retrospective cohort reported a significant association between HPV exposure and fetal death [26]. Overall, fetal death differed significantly between women with HPV infection and those uninfected (aOR, 2.23; 95% CI, 1.14–4.37; I2 = 0%; n = 2) (Figure 6).
Birth Defects
Finally, only 1 population-based case-control study reported on birth defects using the presence of genital warts as HPV exposure proxy [21] and found no association (OR, 1.14; 95% CI, 0.58–2.19) (data not presented in the forest plot because only 1 study reported on birth defects).
Risk of Bias Across Studies
The confunnel plot of studies on PTB and on spontaneous abortions suggested that large and small studies with negative association were seemingly not published (Supplementary Figure 2). Furthermore, the cumulative meta-analyses, sorting studies from the largest to the smallest, suggested the presence of small-study effect because summary OR shifted to the right with the addition of smaller studies (Supplementary Figure 3). The small number of studies for each of secondary outcomes made it impossible to assess the risk of bias across studies and the subgroup analyses.
DISCUSSION
This systematic review and meta-analysis suggest that HPV is associated with PTB, IUGR, LBW, PROM, PPROM, and fetal death. No significant associations were found for spontaneous abortion and PIHDs. However, our findings should be interpreted with caution, given the substantial between-study heterogeneity.
The summary estimates of association between HPV and PTB and remained positive across subgroups and sensitivity analyses. These results give confidence in the association between HPV and PTB. In contrast, HPV exposure was negatively associated with spontaneous abortion in 5 studies [15, 19, 32, 36, 39] and positively associated in 10 studies [5, 16, 18, 25, 27, 33–35, 45, 46]. It is possible that the choice of full-term deliveries as a control group may have negatively biased the association between HPV and spontaneous abortion. The summary estimates were divergent between studies that used full-term deliveries as controls (OR, 0.90; 95% CI, 0.28–2.56) [15, 16, 19, 45, 46] and those that used elective abortions (OR, 1.75; 95% CI, 0.38–7.98) [5, 27, 39] or spontaneous abortions (OR, 1.73; 95% CI, 1.05–2.84) [18, 25, 32–36]. This may support published evidence, suggesting that as gestational age increases, pregnant women are more susceptible to HPV infections. Indeed, the level of progesterone increases steadily throughout pregnancy and stabilizes at approximately the 32nd week of gestation [48]. This downregulates cell-mediated immunity, which is necessary to maintain the fetus, but may also reduce the likelihood of clearance of infections, such as HPV [48]. Therefore, there may be a higher proportion of HPV-exposed women at term delivery than in 1st or 2nd trimester pregnant women who experienced spontaneous abortion. Thus, comparing spontaneous abortions with term deliveries may have underestimated the association between HPV and spontaneous abortion. Moreover, cross-sectional or case-control studies are not suitable to capture early abortions, which are often clinically silent. Indeed, according to in vitro studies, a large part of the negative effects of HPV on pregnancy development would occur early in pregnancy [49].
Estimates for PROM and PPROM are also all positively associated with HPV. For the other secondary adverse pregnancy outcomes, the potential for bias and the small number of published studies prevented firm conclusions.
Participants were recruited from diverse settings and may have differed in terms of age, prevalence of HPV, or baseline risk of adverse pregnancy outcomes. All of these differences likely contributed to the between-study heterogeneity. In most of the studies, adjustment was done for age, the most important potential confounder. Other potential confounders, such as previous adverse pregnancy outcomes or other genital infections, were assessed by only a limited number of studies. Thus, the presence of confounding could have influenced the observed associations.
Strengths
To our knowledge, this is the most comprehensive systematic review assessing the association between HPV and adverse pregnancy outcomes. The review was focused on rigorous analysis of the heterogeneity, assessment of quality of studies, and impact of confounding. Unlike previous reviews, rather than focusing on summary estimates yielded by meta-analyses, we emphasized the patterns and potential causes of heterogeneity between studies.
First, Huang et al [8] found a significant association between HPV exposure and PTB (OR, 2.12; 95% CI, 1.51–2.98; I2 = 61%; n = 8). There was a lack of appreciation for bias that would have been caused by inadequate measurement of HPV exposure in several included studies. Indeed, HPV detection was before pregnancy or postnatal in 3 of 8 studies. According to in vitro studies and animal models [2, 3], one can assume that pregnancy represents the exposure time-window. Thus, considering the high clearance rate of HPV, measuring HPV exposure out of the pregnancy period may bias the associations. We found that the PTB summary estimate was strong and significant when cervicovaginal samples were taken during pregnancy or just after delivery.
Second, Bonde et al [7] conducted a narrative review on the adverse pregnancy outcomes related to HPV without exploration of the between-study variability. In addition to a narrative synthesis, we conducted meta-analyses for different adverse pregnancy outcomes, focusing on the assessment of potential causes of heterogeneity through several subgroup analyses.
The latest review by Ambühl et al [9] summed up HPV prevalence from different studies into 1 single value and compared this global HPV prevalence between women with normal pregnancies to those with adverse pregnancy outcomes. Such a data synthesis approach ignores the weight of each study and the heterogeneity between studies [50]. We have overcome these limitations by computing weighted pooled crude and adjusted estimates using the random-effect model, which accounts for the interstudies variability [11].
Limitations
This systematic review shows that the main limitations of published evidence of the effect of HPV on pregnancy outcomes pertain to the following: (1) within studies - confounding, misclassification of exposure and/or outcomes, and detection of HPV at an inappropriate time-point; and (2) across studies - unexplained heterogeneity and possible publication bias.
There was high heterogeneity for each primary adverse pregnancy outcome, which could be explained to some extent by differences in exposure and/or outcome definitions. Indeed, the PTB pooled estimate increased from 1.50 (95% CI, 1.19–1.88) to 2.01 (95% CI, 1.33–3.03) as a result of restricting analyses to studies that measured HPV exposure by HPV-DNA testing. This suggests that defining HPV exposure on the basis of HPV/Pap cotesting, cytology alone, or presence of condylomas would have led to a nondifferential misclassification of exposure status, contributing to a bias toward the null. Likewise, outcome misclassification may have affected the summary estimates. Although most of studies defined PTB as delivery before 37 weeks of gestation, all except for one [29] did not mention how gestational age was established. In some studies, the PIHDs were merely designated as hypertensive disorders. We assume that if there was any misclassification of adverse pregnancy outcome, it would have been nondifferential and that could have biased the estimate toward the null. Finally, it was impossible to assess the association between specific HPV genotypes and adverse pregnancy outcomes. Most studies provided a measure for presence/absence of HPV or detected a cluster of HPV genotypes.
Only 2 studies compared outcomes according to specific HPV types, and neither was not powered to identify clinically relevant differences in risk of adverse pregnancy outcomes according to HPV type [39, 43].
The heterogeneity persisted within most of subgroups. Even in rare subsets of studies with low heterogeneity (I2 ≤25%), there was a large (95%) uncertainty interval on the variability between studies [11]. Furthermore, meta-regression was not appropriate because there were less than 10 studies for each of the clinical or methodological stratification factors.
CONCLUSIONS AND IMPLICATIONS
This review suggests with fairly high confidence that HPV exposure is associated with PTB. The results also support a possible association between HPV and PROM/PPROM. Although HPV was also associated with IUGR, LBW, and fetal death, the small number of studies and potential for bias prevent firm conclusions for these outcomes. No association were found for spontaneous abortion although this could be explained by the inappropriate definition of comparison groups in some studies. No association was found for PIHD.
Further studies should use HPV tests in pregnancy to define HPV exposure. Adequate control for confounding is also required, given the several common risk factors associated with both HPV and adverse pregnancy outcomes. Moreover, conducting prospective cohorts of women in very early pregnancy would be advantageous for capturing early adverse pregnancy outcomes, such as 1st-trimester miscarriages using an appropriate comparison group. In addition, given the high clearance rate of HPV infection and hormonal-dependent HPV susceptibility during pregnancy, the timing of HPV detection and the need for repeated measurements should be carefully considered. Data from adequately powered studies investigating the impact of specific genotypes of HPV on the risk of adverse pregnancy outcomes are urgently needed. The finding that high- and low-risk HPVs are equally associated with adverse pregnancy outcomes would lend strong support to the development of new broader-spectrum HPV vaccines. This finding would have a major public health impact, because HPV vaccination could decrease the important burden associated with adverse pregnancy outcomes.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Philippe Dodin, the health specialist librarian at Sainte-Justine Hospital (Montreal, Canada), for invaluable contribution to the search strategy development and Louise Laporte for her contribution in the revision of the manuscript.
Financial support. J. N. holds a PhD scholarship from the Quebec Training Network in Perinatal Research. Funding for a PhD award was also provided to J. N. in part by a grant from the Canadian Institutes of Health Research (CIHR) (MOP-136833; to H. T.). H. T. holds a salary award ( Research Scholar) from the Fonds de Recherche du Québec-Santé (FRQ-S) and from CIHR (New Investigator Salary Award). M.-H. M. holds a salary award (Clinical Research Scholar) from the FRQ-S.
Potential conflicts of interest. H.T. has received occasional lecture from Merck and unrestricted grant form ViiV Healthcare. All other co-authors have no conflict of interests.
References
- 1. Nadeau HC, Subramaniam A, Andrews WW. Infection and preterm birth. Semin Fetal Neonatal Med 2016; 21:100–5. [DOI] [PubMed] [Google Scholar]
- 2. Henneberg AA, Patton WC, Jacobson JD, Chan PJ. Human papilloma virus DNA exposure and embryo survival is stage-specific. J Assist Reprod Genet 2006; 23:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hong LJ, Oshiro BT, Chan PJ. HPV-16 exposed mouse embryos: a potential model for pregnancy wastage. Arch Gynecol Obstet 2013; 287:1093–7. [DOI] [PubMed] [Google Scholar]
- 4. Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod 2008; 23:709–15. [DOI] [PubMed] [Google Scholar]
- 5. Hermonat PL, Han L, Wendel PJ, et al. . Human papillomavirus is more prevalent in first trimester spontaneously aborted products of conception compared to elective specimens. Virus Genes 1997; 14:13–7. [DOI] [PubMed] [Google Scholar]
- 6. McDonnold M, Dunn H, Hester A, et al. . High risk human papillomavirus at entry to prenatal care and risk of preeclampsia. Am J Obstet Gynecol 2014; 210:138 e1–5. [DOI] [PubMed] [Google Scholar]
- 7. Bonde U, Joergensen JS, Mogensen O, Lamont RF. The potential role of HPV vaccination in the prevention of infectious complications of pregnancy. Expert Rev Vaccines 2014; 13:1307–16. [DOI] [PubMed] [Google Scholar]
- 8. Huang QT, Zhong M, Gao YF, et al. . Can HPV vaccine have other health benefits more than cancer prevention? A systematic review of association between cervical HPV infection and preterm birth. J Clin Virol 2014; 61:321–8. [DOI] [PubMed] [Google Scholar]
- 9. Ambühl LM, Baandrup U, Dybkær K, Blaakær J, Uldbjerg N, Sørensen S. Human papillomavirus infection as a possible cause of spontaneous abortion and spontaneous preterm delivery. Infect Dis Obstet Gynecol 2016; 2016:3086036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62:e1–34. [DOI] [PubMed] [Google Scholar]
- 11. Niyibizi J, Zanré N, Mayrand MH, Trottier H. The association between adverse pregnancy outcomes and maternal human papillomavirus infection: a systematic review protocol. Syst Rev 2017; 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Effective Public Health Practice Project (EPHPP). Quality Assessment Tool for Quantitative Studies Available at: https://www.nccmt.ca/knowledge-repositories/search/14. Accessed 7 February 2019.
- 13. Slatter TL, Hung NG, Clow WM, Royds JA, Devenish CJ, Hung NA. A clinicopathological study of episomal papillomavirus infection of the human placenta and pregnancy complications. Mod Pathol 2015; 28:1369–82. [DOI] [PubMed] [Google Scholar]
- 14. Hong JN, Berggren EK, Campbell SL, Smith JS, Rahangdale L. Abnormal cervical cancer screening in pregnancy and preterm delivery. Paediatr Perinat Epidemiol 2014; 28:297–301. [DOI] [PubMed] [Google Scholar]
- 15. Ticconi C, Pietropolli A, Fabbri G, Capogna MV, Perno CF, Piccione E. Recurrent miscarriage and cervical human papillomavirus infection. Am J Reprod Immunol 2013; 70:343–6. [DOI] [PubMed] [Google Scholar]
- 16. Conde-Ferráez L, Chan May Ade A, Carrillo-Martínez JR, Ayora-Talavera G, González-Losa Mdel R. Human papillomavirus infection and spontaneous abortion: a case-control study performed in Mexico. Eur J Obstet Gynecol Reprod Biol 2013; 170:468–73. [DOI] [PubMed] [Google Scholar]
- 17. Cho G, Min KJ, Hong HR, et al. . High-risk human papillomavirus infection is associated with premature rupture of membranes. BMC Pregnancy Childbirth 2013; 13:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang R, Wang Y, Qiao J, Liu P, Geng L, Guo YL. Does human papillomavirus infection do harm to in-vitro fertilization outcomes and subsequent pregnancy outcomes? Chin Med J (Engl) 2013; 126:683–7. [PubMed] [Google Scholar]
- 19. Skoczyński M, Goździcka-Józefiak A, Kwaśniewska A. Prevalence of human papillomavirus in spontaneously aborted products of conception. Acta Obstet Gynecol Scand 2011; 90:1402–5. [DOI] [PubMed] [Google Scholar]
- 20. Zuo Z, Goel S, Carter JE. Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. Am J Clin Pathol 2011; 136:260–5. [DOI] [PubMed] [Google Scholar]
- 21. Bánhidy F, Acs N, Puhó EH, Czeizel AE. Birth outcomes among pregnant women with genital warts. Int J Gynaecol Obstet 2010; 108:153–4. [DOI] [PubMed] [Google Scholar]
- 22. Mammas IN, Sourvinos G, Spandidos DA. Maternal human papillomavirus (HPV) infection and its possible relationship with neonatal prematurity. Br J Biomed Sci 2010; 67:222–4. [DOI] [PubMed] [Google Scholar]
- 23. Karowicz-Bilińska A. [The latent infection of human papilloma virus in pregnant woman and colonization of placenta–preliminary report]. Ginekol Pol 2007; 78:966–70. [PubMed] [Google Scholar]
- 24. Torres A, Rosa ER, Méndez K, Menéndez A, Romaguera J. Cervical dysplasia and pre-term birth in San Juan City Hospital: a cohort retrospective study. Bol Asoc Med P R 2013; 105:36–8. [PubMed] [Google Scholar]
- 25. Perino A, Giovannelli L, Schillaci R, et al. . Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril 2011; 95:1845–8. [DOI] [PubMed] [Google Scholar]
- 26. Subramaniam A, Lees BF, Becker DA, Tang Y, Khan MJ, Edwards RK. Evaluation of human papillomavirus as a risk factor for preterm birth or pregnancy-related hypertension. Obstet Gynecol 2016; 127:233–40. [DOI] [PubMed] [Google Scholar]
- 27. Srinivas SK, Ma Y, Sammel MD, et al. . Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol 2006; 195:797–802. [DOI] [PubMed] [Google Scholar]
- 28. Miller ES, Sakowicz A, Grobman WA. The association between cervical dysplasia, a short cervix, and preterm birth. Am J Obstet Gynecol 2015; 213:543 e1–4. [DOI] [PubMed] [Google Scholar]
- 29. Mosbah A, Barakat R, Nabiel Y, Barakat G. High-risk and low-risk human papilloma virus in association to spontaneous preterm labor: a case-control study in a tertiary center, Egypt. J Matern Fetal Neonatal Med 2018; 31:720–5. [DOI] [PubMed] [Google Scholar]
- 30. Cohen E, Levy A, Holcberg G, Wiznitzer A, Mazor M, Sheiner E. Perinatal outcomes in condyloma acuminata pregnancies. Arch Gynecol Obstet 2011; 283:1269–73. [DOI] [PubMed] [Google Scholar]
- 31. Zaidi NI, McNamara JM, Ismail M, Kay HH. Human papilloma virus (HPV) and pregnancy outcomes. Reprod Sci 2010;17(Suppl 3):185A–6A. [Google Scholar]
- 32. López-Hernández D, Beltrán-Lagunes L, Brito-Aranda L, López-Hernández Mde L. [Human papillomavirus infection and its correlates with clinically relevant gynecological and obstetric conditions: a cross-sectional study]. Med Clin (Barc) 2016; 147:101–8. [DOI] [PubMed] [Google Scholar]
- 33. Sikström B, Hellberg D, Nilsson S, Brihmer C, Mårdh PA. Contraceptive use and reproductive history in women with cervical human papillomavirus infection. Adv Contracept 1995; 11:273–84. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka H, Karube A, Kodama H, Fukuda J, Tanaka T. Mass screening for human papillomavirus type 16 infection in infertile couples. J Reprod Med 2000; 45:907–11. [PubMed] [Google Scholar]
- 35. Comar M, Monasta L, Zanotta N, Vecchi Brumatti L, Ricci G, Zauli G. Human papillomavirus infection is associated with decreased levels of GM-CSF in cervico-vaginal fluid of infected women. J Clin Virol 2013; 58:479–81. [DOI] [PubMed] [Google Scholar]
- 36. Oborna I, Ondryasova H, Zborilova B, Brezinova J, Vrbkova J. Does presence of human papillomavirus (HPV) infection influence the results of in vitro fertilization (IVF) treatment? Fertil Steril 2016; 106:e335–6. [Google Scholar]
- 37. Spandorfer SD, Bongiovanni AM, Fasioulotis S, Rosenwaks Z, Ledger WJ, Witkin SS. Prevalence of cervical human papillomavirus in women undergoing in vitro fertilization and association with outcome. Fertil Steril 2006; 86:765–7. [DOI] [PubMed] [Google Scholar]
- 38. Nimrodi M, Kleitman V, Wainstock T, et al. . The association between cervical inflammation and histologic evidence of HPV in PAP smears and adverse pregnancy outcome in low risk population. Eur J Obstet Gynecol Reprod Biol 2018; 225:160–5. [DOI] [PubMed] [Google Scholar]
- 39. Ambühl LMM, Leonhard AK, Widen Zakhary C, et al. . Human papillomavirus infects placental trophoblast and Hofbauer cells, but appears not to play a causal role in miscarriage and preterm labor. Acta Obstet Gynecol Scand 2017; 96:1188–96. [DOI] [PubMed] [Google Scholar]
- 40. Ford JH, Li M, Scheil W, Roder D. Human papillomavirus infection and intrauterine growth restriction: a data-linkage study. J Matern Fetal Neonatal Med 2019; 32:279–85. [DOI] [PubMed] [Google Scholar]
- 41. Kaur H. Does human papillomavirus affect pregnancy outcomes? An analysis of hospital data 2012–2014. Int J Women’s Health Wellness 2015;1. [Google Scholar]
- 42. Caballero A, Dudley D, Ferguson J, Pettit K, Boyle A. Maternal human papillomavirus and preterm premature rupture of membranes: a retrospective cohort study. J Women’s Health (Larchmt) 2019; 28:606–11. [DOI] [PubMed] [Google Scholar]
- 43. Aldhous MC, Bhatia R, Pollock R, et al. . HPV infection and pre-term birth: a data-linkage study using Scottish health data. Wellcome Open Res 2019; 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vyankandondera J, Wambua S, Irungu E, et al. . Type-specific human papillomavirus prevalence, incident cases, persistence, and associated pregnancy outcomes among HIV-infected women in Kenya. Sex Transm Dis 2019; 46:532–9. [DOI] [PubMed] [Google Scholar]
- 45. Bober L, Guzowski G, Moczulska H, Sieroszewski P. Influence of human papilloma virus (hPV) infection on early pregnancy. Ginekol Pol 2019; 90:72–5. [DOI] [PubMed] [Google Scholar]
- 46. Pandey D, Solleti V, Jain G, et al. . Human papillomavirus (HPV) infection in early pregnancy: prevalence and implications. Infect Dis Obstet Gynecol 2019; 2019:4376902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaur H, Schmidt-Grimminger D, Chen B, et al. . HPV prevalence and its association with perinatal outcomes among singleton mothers: analysis of pregnancy risk assessment and monitoring system (PRAMS) data, 2004–2011. Current Women’s Health Reviews 2019; 15:143–9. [Google Scholar]
- 48. Banura C, Franceschi S, van Doorn LJ, et al. . Prevalence, incidence and clearance of human papillomavirus infection among young primiparous pregnant women in Kampala, Uganda. Int J Cancer 2008; 123:2180–7. [DOI] [PubMed] [Google Scholar]
- 49. Noventa M, Andrisani A, Gizzo S, Nardelli GB, Ambrosini G. Is it time to shift the attention on early stages embryo development to avoid inconclusive evidence on HPV-related infertility: debate and proposal. Reprod Biol Endocrinol 2014; 12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deeks JJ. Systematic reviews of published evidence: miracles or minefields? Ann Oncol 1998; 9:703–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.