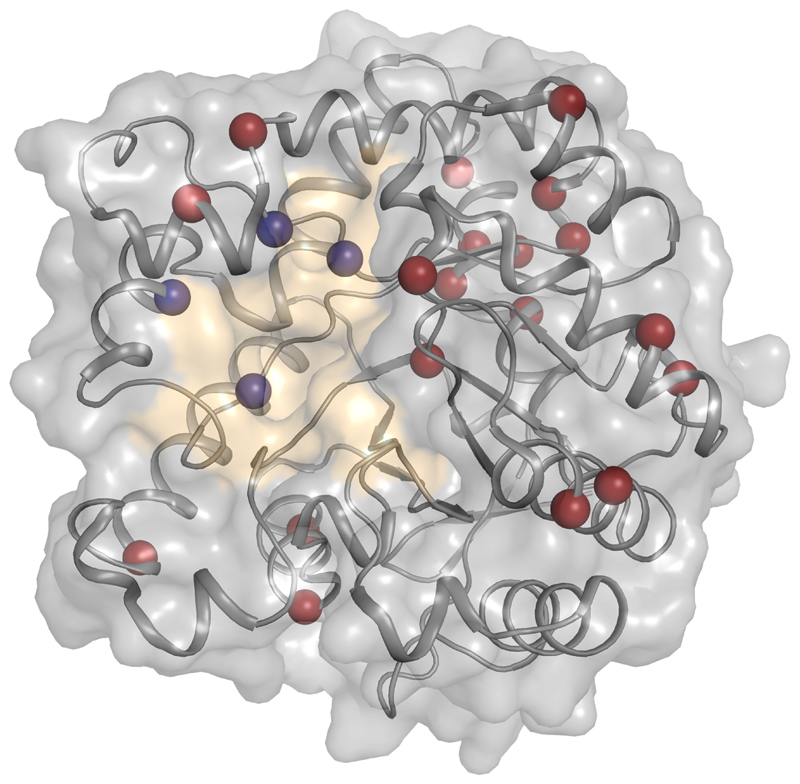
Figure 2. A practical two-step design strategy for enzyme optimization.
A stable and high-efficiency esterase was designed first by the PROSS stability-design method[33], which introduced 19 mutations in positions remote from the active site (red spheres) and then by the FuncLib function-enhancing method, which introduced four active-site mutations[43] (the active site is surface-colored in yellow and the FuncLib mutations are marked in blue spheres). This design exhibited higher stability, bacterial expression levels and approximately 1,000-fold higher esterase activity relative to the parental enzyme. Other enzymes reported in this study exhibited nearly 4,000-fold improvement in the hydrolysis of venomous nerve agents. This first stabilization step may address the stability-threshold challenge by improving the enzyme’s tolerance for function-enhancing mutations in the second step. For clarity, only one of the two chains comprising the enzyme is shown here.