Introduction
The unprecedented demand for hospital services during the SARS-CoV-2 (COVID-19) pandemic has dramatically reduced the capability for dealing with non-acute health needs, including cancer care [1, 2]. To alleviate burden on health care systems, including imaging and laboratory services, curtailment of non-COVID-19-related research activity has been necessary [3]. Measures that reduce hospital visits have been adopted to limit risk of infection and death, which is critical in a cancer population whose age and immunocompromised status increases their risk [4]. Imaging, however, requires hospital visits and close contact with staff and equipment; both are sources of disease transmission. Equipment used to image COVID-19 cases may retain virus on its surface for days [5, 6] unless disinfected. The need for social distancing and for disinfecting equipment substantially slows imaging workflow and reduces throughput. This article discusses the specific impact of pandemics such as SARS-CoV-2 on imaging in oncological trials.
Patients currently on trials
Imaging trial activities that demand quality assurance, technical and biological validation and PET pharmacokinetic studies are all considered non-essential during a pandemic and are largely discontinued. For ongoing therapeutic trials requiring imaging, a pandemic poses significant challenges. Unlike other adaptations to trial workflow through remote study visits, delayed site-monitoring visits and direct delivery of investigational products to patients who are self-isolating [7], imaging cannot be performed remotely. International pharmaceutical regulatory bodies have not stipulated specific modifications regarding the use of imaging; they merely recommend assessing risks of physical visits, doing study-related assessments at different sites and providing patients with personal protective equipment (PPE) to ensure their safety [8]. As the risk of infection with SARS-CoV-2 to cancer patients attending hospital might be doubled [9], and with many patients fearful of attending hospital even for clinical care, the risk of losing imaging data from oncological trials is high. Dedicated scanning facilities restricted to patients tested negative for infection would provide a potential solution.
The imposed changes on the availability of imaging for trial activity, including the availability of radionuclides and radiopharmaceuticals, and the risks of infection with hospital attendance mean that principal investigators and sponsors may re-evaluate imaging frequency within existing trial protocols. However, a reduction in imaging frequency may miss milestones that indicate progression or harmful side effects and continuation on an ineffective or toxic drug regimen. Postponing the acquisition of imaging-based trial endpoints also jeopardises timely recording of progression events, which can distort estimates of treatment effect and lead to erroneous trial conclusions (e.g., efficacious intervention but negative trial). In fact, it may be argued that imaging frequency should be increased to monitor complications (pneumonitis, pulmonary embolism), posed by the additional risks of COVID-19 in pyrexial trial patients [10].
A major challenge also remains in image interpretation. Differentiation of pandemic-specific (COVID-19) pulmonary changes from other pneumonias requires definitive molecular testing [10]. When a patient drops out of a trial due to infection, there are implications for ongoing patient management and for trial endpoints.
New patient recruitment
The abrupt discontinuation of research activities due to a pandemic such as COVID-19 also has had a substantial impact on new recruitment of patients into oncological trials (Fig. 1). Risk of secondary infection in immunocompromised cancer patients and diversion of radiology, pathology and pharmacy services to cope with the pandemic has meant that many sponsors have deferred new patient recruitment. Moreover, the reduction in non-urgent hospital visits has reduced the number of new cancer diagnoses [1] and the opportunity to evaluate the eligibility and suitability of potential new recruits, based on imaging. Capacity to perform image-guided biopsies which are crucial in determining trial eligibility also can be limited by radiologist, facilities and PPE shortages.
Fig. 1.
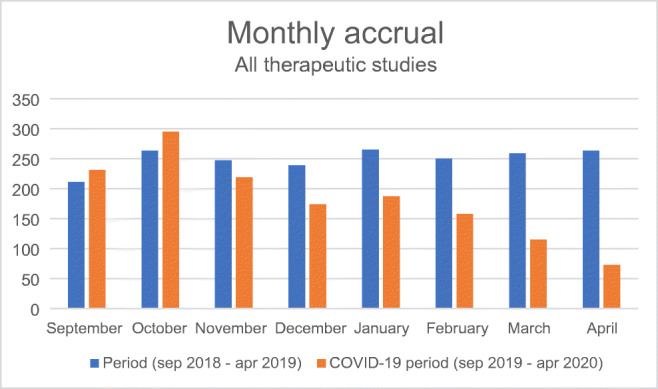
Total accrual to EORTC therapeutic studies in the four months prior and 4 months during COVID-19 pandemic, compared to the total recruitment over the corresponding months 1 year before
Baseline imaging findings often affect inclusion of new patients, by increasing the need for additional imaging to evaluate incidental findings and by requiring complementary modalities to ensure inclusion criteria are met. In the presence of unexpected COVID-19-specific pneumonia on baseline imaging, managing the patient and potentially recruiting them to newly developed COVID-19 trials has taken priority over oncology trials. Treatments such as chimeric antigen receptor (CAR) T-cells, often done in a trial setting, must be avoided if there is active SARS-CoV-2 infection. As restrictions ease, and hospital visits recommence, options to increase recruitment to oncological trials include remote visits and video-based communication to inform potential candidates about trials and maintain productivity [11]. However, baseline imaging and biopsies to establish eligibility will require hospital visits, which may well be still considered low priority. Delays in recruiting patients risk their oncological progression and subsequent ineligibility. Trials that require multiple image-guided biopsies whilst on treatment may be particularly difficult to re-start because of capacity issues. Initiation of new projects (“site initiation visit”) is deferred until travel restrictions are lifted and visits to hospitals by non-essential persons are restored.
Patient perspective
Participation in clinical trials is profoundly dictated by a patient’s perception of the potential risk-benefit to themselves [12], and imaging may be perceived as a burden [13]. Many imaging-based clinical trials, however, merely incorporate research imaging as an additional component to routine imaging or utilise images acquired for clinical care. The impact of a pandemic in these cases therefore reflects that on non-essential clinical imaging services [14]. In therapeutic trials with multiple imaging studies at intervals, patients may be hesitant to participate because of concerns, fuelled by official regulations and messages, over frequent hospital visits and reluctance to use public transport without adequate PPE. For patients living in communities or institutions, imposed quarantine periods on return from hospital are another deterrent.
After a pandemic, oncology and imaging departments need to jointly rebuild patient confidence in trial visits. Where available and practical, dedicated “infection-cold” care areas with separate patient pathways and scanner units could be designated and this communicated to trial patients. Contacting patients prior to the imaging visit, enquiring about possible symptoms, should reassure them that safety procedures are enforced and that imaging examinations can be re-scheduled where needed. Additional body temperature checks at the entrance of scanning facilities, supply of surgical masks, provision for hand sanitisation, social distancing measures in waiting areas, signage and one-way systems through imaging departments [14, 15] all inspire confidence in patients attending for trial imaging studies.
Waiting times for scan results are a known source of patient anxiety [16]. The pandemic has forced an upsurge in telemedicine communication pathways, so exploiting these to maintain patient motivation for trial imaging attendance could be used to advantage. Moreover, as imaging studies may potentially reveal incidental findings suggestive of pandemic-related (COVID-19) infection, information from structured and timely review of imaging findings communicated early to patients within trials would further incentivise them to attend imaging sessions. Patients should be made aware of these advantages in monitoring and care that support their trial participation.
Organisational issues—institution and department policies
Trial-related imaging, currently delayed due to any “lockdown” strategy adopted in most countries and pandemic-related priorities, is delayed further by post-pandemic imaging capacity limits. Social distancing measures are likely to remain imposed after lifting a lockdown. Once routine practice is resumed, a certain period of re-adjustment is needed to cope concurrently with new and legacy workload in already tight healthcare systems. Imaging within clinical trials is often low priority, particularly in smaller centres where spare imaging capacity is non-existent. In trials where imaging is considered optional, or where imaging is the sole research question, image data collection might suffer.
Workflow within imaging departments is likely to be adversely affected by the need for using PPE, particularly for procedures such as ultrasound and image-guided biopsies. This has implications for time, preparation and waiting room space. Examinations will take longer and, where protocols require adaptation, their complexity will increase. It is essential that the workflow through an imaging department is optimised for the clinical workload to effectively accommodate imaging for trials.
The increase in reporting workload for nuclear medicine physicians and radiologists when non-urgent clinical work re-starts may well also impact the timely scoring and reporting of trial data. The consequences of pandemic-induced stress on imaging staff in a post-pandemic era should not be underestimated [17], especially when they have been re-deployed to the “front line”. Where incidental findings of pandemic-related (COVID-19) infection are noted in trial patients, rapid communication channels to site principal investigators should be established.
COVID-19 pneumonia as an ‘incidental’ finding
Many oncological patients undergo chest CT to assess their primary tumour or to rule out lung metastasis. The lungs also are partially included on non-chest dedicated CTs, e.g. H&N, abdomen, CT for SPECT/CT or on daily radiotherapy cone beam CT scans [18]. Where abnormality is noted, a full-chest CT is warranted. Therefore, patients on oncological trials will inevitably have incidentally detected findings that could be attributable to COVID-19 pneumonia. A 9% incidence was reported from a nuclear medicine department performing routine [18F]-FDG-PET/CT and SPECT/CT in oncology patients during the peak of the pandemic [19]. Patterns of COVID-19 pneumonias with these imaging modalities are now emerging [20] and where detected should prompt an immediate alert and SARS-COV-2 testing. The need for supplementary dedicated chest CT in (a)symptomatic patients would follow similar principles regardless of the pandemic. However, the requirement for radiologists to decide whether or not to mention COVID-19 specifically, rather than simply “viral” pneumonia, is debatable. Expert consensus statements for reporting chest CT findings of COVID-19 have been published [21, 22]. From a trial perspective, it remains essential only to distinguish infection/inflammation from oncological progression. From an imaging perspective, how these pneumonias are recorded influences the assessment of the specific impact of the COVID-19 pandemic on trial outcome. From a public health perspective, these patients need to be isolated.
Opportunities
For the reasons discussed, patients might miss imaging follow-up visits during oncology trials, which will create subpopulations of patients with strictly trial-governed versus limited imaging follow-up. This offers the opportunity for a critical review of the real need for and frequency of imaging. While imaging follow-up through cancer treatment is a pillar of oncology trials to monitor treatment efficacy and detect recurrences, it is recognised that recommendations in terms of frequency of follow-up are often empirical/arbitrary, particularly if there is no evidence of disease. Previous work showed that intensifying follow-up of cancer patients did not improve overall survival nor influence health-related quality of life [23, 24]. This period of reduced imaging availability could be an opportunity for (re)discovering the true value of imaging, rather than merely exploiting it for reassurance and surveillance.
Future perspective
Based on prior experience with SARS and MERS, COVID-19 is unlikely to be the last pandemic or major global crisis that impacts imaging for patient care or in clinical trials. Accordingly, there are important lessons to be learned from the shortcomings and experience of the current situation. With imaging so integral to clinical trials in oncology, it is crucial that cancer research is not held back by failure to deliver timely imaging, compliant to protocol. Pathways where imaging studies can be directed to local sites with spare capacity, semi-automated readouts to reduce staff pressure and facilities that exclude potentially infectious patients need to be within institutional policies, to pre-empt and minimise disruption to imaging within research trials in future pandemics [25]. Ongoing adaptations could be the creation of safer pathways, dedicated to vulnerable patients within imaging units and hospitals and minimisation of potential exposure to pathogens through grouped clinical, laboratory and imaging visits. Flexible imaging protocols, adaptable strategies and response measures [26–28] to account for differences in national healthcare organisation and in line with the national severity of the crisis should enable the imaging community to deal with future events with minimal disruption and maximal efficiency so as to deliver time-dependent and time-critical studies within clinical trials.
Conclusion
Re-instatement of normal imaging workload after a pandemic [29] requires administrative support and planning adaptation, with extended scanning hours and appointments through a workflow that retains social distancing practices and incorporates additional hygiene processes. A reduced throughput for clinical imaging studies will inevitably impact trial-related imaging and image-guided biopsies, resulting in a slowdown of trial activity. To counteract this, the lessons learned regarding smarter use of imaging within trials and improved communications systems between physicians and imagers, doctors and patients should be exploited to improve the delivery of imaging within oncological trials in future crises.
Total accrual to EORTC therapeutic studies in the 4 months prior and 4 months during COVID-19 pandemic compared to the total recruitment over the corresponding months 1 year before.
Funding information
This publication was supported by a donation from “Kom op tegen Kanker” from Belgium. Christophe M. Deroose is a Senior Clinical Investigator at Research Foundation—Flanders (FWO). No writing assistance was obtained for this manuscript.
Compliance with ethical standards
Disclaimer
The funding sources had no active role in collection of the data, writing and final approval of the manuscript.
Conflict of interest
Christophe M. Deroose reports fees for consulting or advisory roles with Ipsen, Novartis, Terumo and Advanced Accelerator Applications; participation in speakers’ bureaus with Terumo and Advanced Accelerator Applications and travel, accommodations or expenses with General Electric and Terumo.
Caroline Caramella reports honoraria from Pfizer, BMS, MSD, Astra-Zeneca and Roche.
Laure Fournier reports research support from Philips, ArianaPharma, Evolucare and Invectys; honoraria or consulting fees from Novartis, Janssen and Sanofi and speaker fees from Novartis, Bayer, Janssen, Sanofi, Pfizer and GE Healthcare.
Marion Smits reports honoraria paid to the institution for trial review by Parexel Ltd. and for lecturing by GE Healthcare.
Laurence Collette, Daniela E. Oprea-Lager, Wolfgang G. Kunz, Frédéric Lecouvet, Luc Bidaut, Joost J.C. Verhoeff, Egesta Lopci, Bertrand Tombal, Lioe-Fee de Geus-Oei, Laure Fournier and Nandita M. deSouza declare no conflicts of interest.
Studies with human participants or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Oncology General.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020. 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed]
- 2.van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26:665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 3.Gewin V. Safely conducting essential research in the face of COVID-19. Nature. 2020;580:549–550. doi: 10.1038/d41586-020-00896-7. [DOI] [PubMed] [Google Scholar]
- 4.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020. 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed]
- 5.Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6:e01697–e01615. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan AC, Ashley DM, Khasraw M. Adapting to a pandemic - conducting oncology trials during the SARS-CoV-2 pandemic. Clin Cancer Res. 2020. 10.1158/1078-0432.CCR-20-1364. [DOI] [PMC free article] [PubMed]
- 8.de Paula BHR, Araujo I, Bandeira L, Barreto N, Doherty GJ. Recommendations from national regulatory agencies for ongoing cancer trials during the COVID-19 pandemic. Lancet Oncol. 2020;21:624–627. doi: 10.1016/S1470-2045(20)30226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed]
- 10.Yang W, Sirajuddin A, Zhang X, Liu G, Teng Z, Zhao S, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur Radiol. 2020. 10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed]
- 11.Vagal A, Reeder SB, Sodickson DK, Goh V, Bhujwalla ZM, Krupinski EA. The impact of the COVID-19 pandemic on the radiology research Enterprise: radiology scientific expert panel. Radiology. 2020;201393. 10.1148/radiol.2020201393. [DOI] [PMC free article] [PubMed]
- 12.Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105–114. doi: 10.1200/EDBK_243729. [DOI] [PubMed] [Google Scholar]
- 13.May K, Lee M, Jefford M, Ribeiro A, Macdonald A, Morgan V, et al. Imaging in clinical trials: a patient-led questionnaire study to assess impact of imaging regimes on patient participation. Res Involv Engagem. 2020;6:15. doi: 10.1186/s40900-020-00195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP, Tan BS. Radiology department preparedness for COVID-19: radiology scientific expert panel. Radiology. 2020;200988. 10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed]
- 15.Goh Y, Chua W, Lee JKT, Ang BWL, Liang CR, Tan CA, et al. Operational strategies to prevent coronavirus disease 2019 (COVID-19) spread in radiology: experience from a Singapore radiology department after severe acute respiratory syndrome. J Am Coll Radiol JACR. 2020. 10.1016/j.jacr.2020.03.027. [DOI] [PMC free article] [PubMed]
- 16.Ollivier L, Apiou F, Leclere J, Sevellec M, Asselain B, Bredart A, et al. Patient experiences and preferences: development of practice guidelines in a cancer imaging department. Cancer Imaging. 2009;9 Spec No A:S92–S97. [DOI] [PMC free article] [PubMed]
- 17.Giovagnoni A. Facing the COVID-19 emergency: we can and we do. La Radiologia medica. 2020;125:337–338. doi: 10.1007/s11547-020-01178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youssef IDB, Flyer M, Thompson S, Huang A, Gallant F. Covert Covid-19: CBCT lung changes in an asymptomatic patient receiving radiotherapy. Adv Radiat Oncol. 2020. https://www.astro.org/ASTRO/media/ASTRO/Daily%20Practice/PDFs/COVID-Youssef(ADRO).pdf. [DOI] [PMC free article] [PubMed]
- 19.Albano D, Bertagna F, Bertoli M, Bosio G, Lucchini S, Motta F, et al. Incidental findings suggestive of COVID-19 in asymptomatic patients undergoing nuclear medicine procedures in a high-prevalence region. J Nucl Med. 2020;61:632–636. doi: 10.2967/jnumed.120.246256. [DOI] [PubMed] [Google Scholar]
- 20.Tulchinsky M, Fotos JS, Slonimsky E. Incidental CT Findings suspicious for Covid-19 associated pneumonia on nuclear medicine exams: recognition and management plan. Clin Nucl Med 2020. 10.1097/RLU.0000000000003100. [DOI] [PMC free article] [PubMed]
- 21.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020. 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed]
- 22.Prokop M, van Everdingen W, van Rees VT, Quarles van Ufford J, Stoger L, Beenen L, et al. CO-RADS - a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020:201473. 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed]
- 23.Benamore R, Shepherd FA, Leighl N, Pintilie M, Patel M, Feld R, et al. Does intensive follow-up alter outcome in patients with advanced lung cancer? J Thorac Oncol. 2007;2:273–281. doi: 10.1097/01.JTO.0000263708.08332.76. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200. doi: 10.1002/14651858.CD002200.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davenport MS, Bruno MA, Iyer RS, Johnson AM, Herrera R, Nicola GN, et al. ACR statement on safe resumption of routine radiology care during the COVID-19 pandemic. J Am Coll Radiol. 2020. 10.1016/j.jacr.2020.05.001. [DOI] [PMC free article] [PubMed]
- 26.Paez D, Gnanasegaran G, Fanti S, Bomanji J, Hacker M, Sathekge M, et al. COVID-19 pandemic: guidance for nuclear medicine departments. Eur J Nucl Med Mol Imaging. 2020. 10.1007/s00259-020-04825-8. [DOI] [PMC free article] [PubMed]
- 27.Zhao Y, Xiang C, Wang S, Peng C, Zou Q, Hu J. Radiology department strategies to protect radiologic technologists against COVID19: experience from Wuhan. Eur J Radiol. 2020;127:108996. doi: 10.1016/j.ejrad.2020.108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czernin J, Fanti S, Meyer PT, Allen-Auerbach M, Hacker M, Sathekge M, et al. Nuclear medicine operations in the timeS of COVID-19: strategies, precautions, and experiences. J Nucl Med. 2020;61:626–629. doi: 10.2967/jnumed.120.245738. [DOI] [PubMed] [Google Scholar]
- 29.Luker GBA. Transitioning to a new normal after COVID-19: preparing to get back on track for cancer imaging. Radiol Imaging Cancer. 2020. 10.1148/rycan.2020204011. [DOI] [PMC free article] [PubMed]


