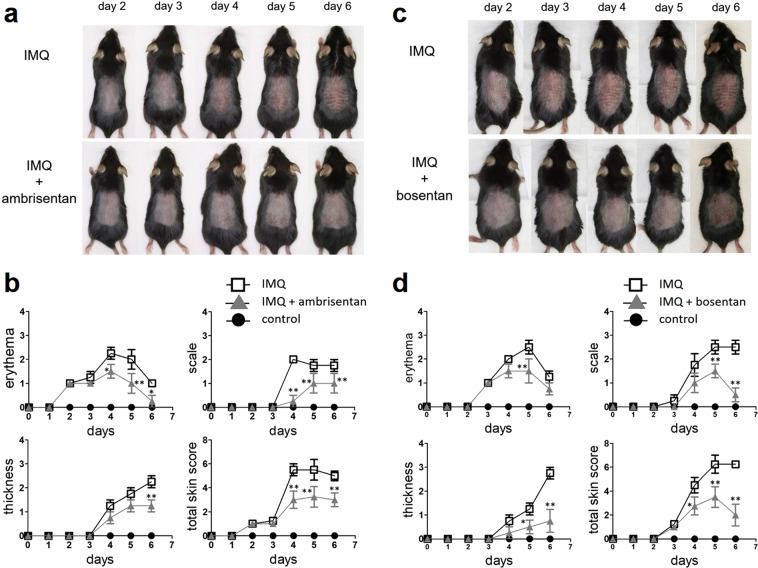
Figure 2.
The effects of topical application of ambrisentan or bosentan on clinical findings of IMQ-induced psoriasiform dermatitis. Shaved back skin and ears of B6 mice were topically treated with IMQ or control vehicle for 6 consecutive days. Topical ambrisentan or bosentan was administered from 4 days before IMQ application until the end of the study. (a,c) Pictures of mice were taken and the phenotypic symptoms of mouse skin were observed from day 0 to day 6. (b,d) Clinical scores for disease severity were calculated daily using a scoring system based on the clinical Psoriasis Area and Severity Index. Erythema, scales, and thickness were scored independently on a scale from 0 to 4: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked. The cumulative score (erythema, scales, and thickness) served as a measure of the severity of inflammation (scale 0–12). Results are representative of similar results obtained in three independent experiments. Data are presented as mean ± SEM (n = 5 for each group). *P < 0.05, **P < 0.01 versus IMQ-treated group.